 Your new post is loading...

|
Scooped by
rob halkes
October 9, 2013 3:26 AM
|
Pharmacists in Europe have backed plans to improve access to clinical trial results in order to allow independent analysis of drug data. The European Association of Hospital Pharmacists, which represents pharmacist organisations from 34 countries in Europe, gave its support to the European Medicines Agency (EMA) policy proposal during a consultation period that ended at the end of last month. The EAHP said that greater transparency was needed to offer opportunities for independent scrutiny of the methodology and results of trials. Improved transparency would also prevent duplication of research and enhance patient safety by increasing knowledge about adverse events, said the EAHP. .. The EMA's proposed policy divides clinical data into three categories of access: commercially confidential information; open-access data that does not contain patients' personal information; and controlled-access data that may include personal data and will only be accessible after the requester fulfils certain requirements. Depending on what category data fits under, individuals and organisations will have controlled access to trial information if they meet certain criteria. Plans for greater transparency have been an increasing subject of interest for pharma in recent years, and have attracted criticism from the European Federation of Pharmaceutical Industries & Associations (EFPIA), concerned by the impact any change would have on commercial confidentiality. .. The EMA said it will consider all comments as it prepares to finalise its policy, which is expected to come into force on January 1, 2014.
Social media is part of our culture. From sharing pictures, updates, and professional networking, to engaging customers and organizing political movements, there is no question about the power of social media. Interestingly, social media is still in its infancy and experts believe that the true potential and value of social media has not been tapped. Social media empowers everyone to share their ideas, amplify their voice, and shape the world. Healthcare professionals are using social media to share healthcare knowledge, advance patient care, and influence their professions. We profile 3 pharmacists actively using social media to build their businesses and advance the pharmacy profession. They are actively participating in the social economy.
Via Marie Ennis-O'Connor

|
Scooped by
rob halkes
October 2, 2013 8:02 AM
|
European Union agency responsible for the protection of public and animal health through the scientific evaluation and supervision of medicines. The European Union (EU) has introduced a new process to label medicines that are being monitored particularly closely by regulatory authorities. These medicines are described as being under 'additional monitoring'. Medicines under additional monitoring have a black inverted triangle displayed in their package leaflet and in the information for healthcare professionals called the summary of product characteristics, together with a short sentence explaining what the triangle means: This medicinal product is subject to additional monitoring. The black triangle will be used in all EU Member States to identify medicines under additional monitoring. It will start appearing in the package leaflets of the medicines concerned from the autumn of 2013. It will not appear on the outer packaging or labelling of medicines. What does the black triangle mean? All medicines are carefully monitored after they are placed on the EU market. If a medicine is labelled with the black triangle, this means that it is being monitored even more intensively than other medicines. This is generally because there is less information available on it than on other medicines, for example because it is new to the market or there is limited data on its long-term use. It does not mean that the medicine is unsafe.

|
Scooped by
rob halkes
September 20, 2013 10:10 AM
|
Russian Conditions to the health care market – How could the industry adapt? This spring, I was as lucky to be invited to the Russian Pharmaceutical Phorum, May 23rd, in St. Petersburg (Adam Smith Conferences ). In the preparation to my key note speech I prepared myself to learn the health care conditions of Russia. .. It is not your intentions that makes the difference to your customers, as well as the actual market activities you deploy will do. If you put your energy into promotional actions, you will be seen as a commercial company, as sales persons. But, to the contrary, when you work with health care professionals’ attempts to create better care for patients, then you can get to be seen as a partner to care, as a colleague maybe.
It relates to your business directly: when you only sell medicine, your business coincides with the price of your product. But when you work together with stakeholders to care for patients you might be a collaborator and your value will go beyond your product’s price. ..

|
Scooped by
rob halkes
July 23, 2013 1:55 PM
|
The industry's innovation crisis will not be solved by quick fixes. Reconfigured value chains will give rise to new business models.
A cure for the pharmaceutical industry's innovation crisis has yet to be found. During the past decade, new molecules output decreased steadily from ~30 NCE per year to ~25 NCE per year while R&D costs more than doubled.1 Many of the attempted remedies may even be responsible for the high attrition rate of pharmaceutical R&D projects. Open innovation, currently trapped in an unsustainable coexistence of both end-to-end in-house capacities and extensive partnering, has yet to deliver its promise. What will future pharma business models look like? In this study we take a fresh look, with a focus on the R&D value chain, and offer five key insights gained from the study's analyses:
1. Quick fixes, such as outsourcing R&D services, have not saved productivity.
2. Constant changes in R&D portfolio prioritization reduce creativity—and destroy pipeline value.
3. There is a clear breakpoint in the R&D value chain between front-end discovery and late-stage development.
4. Pharma value-chain reconfiguration will result in two main new business models:
- “discover modules” - “Iplement therapies”.
5. Pharmaceutical companies will need to actively select and sharpen their business model before uncontrollable market forces drive the change.
Pharma companies that focus on one of the two business models will stand the best chance of success—trying to adopt both at the same time will invite a high risk of failing in both. The business model decision should be guided by a clear understanding of their competitiveness along the value chain, and its implementation should be based on sound, individual strategy. Only by adhering to these principles will a compelling equity story—a prerequisite for profitable future growth—be created.

|
Scooped by
rob halkes
July 19, 2013 5:46 AM
|
There are 5 main areas of Big Data investigation in real world outcomes by pharma today... Remote, mobile health – The use of mobile devices such as smart phones to gather data on treatment adherence, on-going patient care, predictive symptoms or adverse events and health outcomesHealthcare provider data – Collecting data from electronic medical records that includes patient diagnosis, treatment, adherence and outcome.Connecting genomic analysis with patient outcomes and subpopulations in order to progress personalized medicineUtilizing data both from health records and pre-clinical studies to find new indications for therapeutic pipelinesBetter clinical decision making in order to improve patient outcomes with prescribed therapeutic I often see articles that describe how big data won’t save pharma but smart analytics on data might. In the end Big Data is only as good as the questions that are being asked of it and essentially at present Big Data in healthcare is one big Petri dish. Certainly we will see a lot more business intelligence and analytics roles – both in pharma and providers – moving into big data applications and there will be huge demand for this talent in the years to come. This is the time to debate and discuss where the whole strategy of Big Data is going to take us and I hope this ebook inspires your team to invest further in the area. ... See how health care is a "late runner up":

|
Scooped by
rob halkes
July 18, 2013 1:56 PM
|
Within the discussion around AstraZeneca's branded approach to YouTube, I've yet to see any commentary focusing on the fact that whilst the AstraZeneca’s Health Connections blog states that ‘AstraZeneca believes that it is important to share information with patients by engaging with them online’, the principle information it seems to want to convey is the cost of the medication. If I didn’t live in a country with a national health system and I was considering taking Nexium, I’d want to know about its efficacy as a treatment for acid reflux before I turned my attention to how much it costs.
What’s the key message here?
Nexium costs $18 a month. Thanks. Now, what is it for, what does it do, and is it effective? By all means make the implicit message that ‘Nexium is an affordable medication’ explicit, but not at the expense of explaining why the medication is an effective treatment for the conditions it is indicated for. Not doing so makes it look like AstraZeneca is trying to smuggle a message about affordability in through the back door. The truly disappointing thing is, there’s really no need to do this: of course cost is an issue to consumers in geographies that don’t have national health systems, but why not state that plainly rather than embedding the message in a graphic?
AstraZeneca should take a long, cold look at the disjuncture between the narrative that they appear to believe they’re constructing around supporting educational need on their branded channel on YouTube (with comments currently switched off, I notice), and the primacy of the message they’re actually conveying around medication cost which consumers are exposed to first.....

|
Scooped by
rob halkes
June 7, 2013 8:59 AM
|
A lack of confidence about social media is preventing pharmaceutical companies from making wider use of social media, according to a new report. Digital Health: Building Social Confidence in Pharma by PR firm Weber Shandwick also found that regulatory restrictions were no longer seen as the primary barrier to social engagement. Instead the pharma marketing and communications executives interviewed for the report said they faced a greater challenges internally when trying to 'socialise' their strategies, instill social media confidence in their teams and align resources. .. read further at the blog for inspiring quotes! See also the blog for download referal for the new report..

|
Scooped by
rob halkes
June 7, 2013 8:37 AM
|

|
Scooped by
rob halkes
May 31, 2013 5:20 AM
|
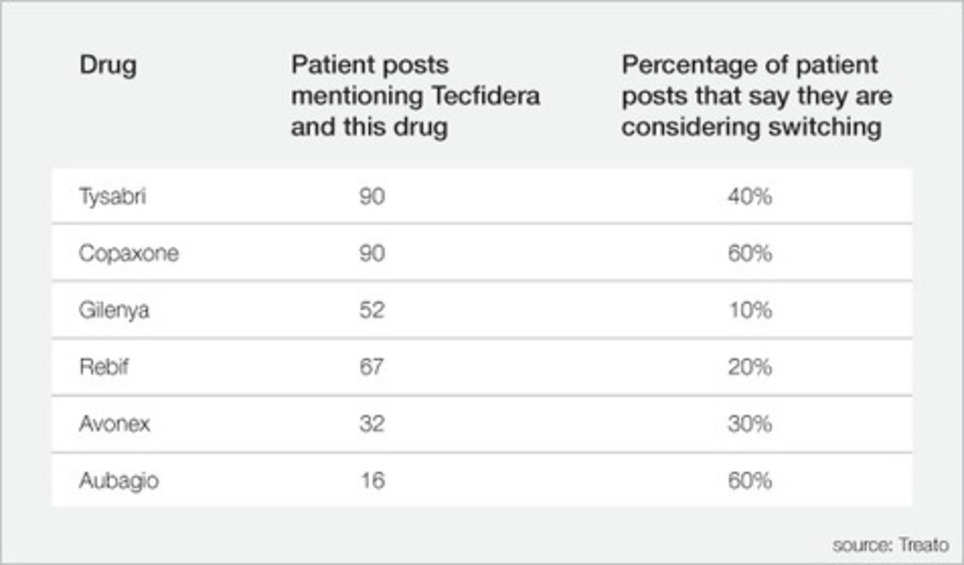
Treato's Gideon Mantel writes: Social media can predict the success of a new drug launch much faster than traditional methods. Many pharmaceutical companies try to measure the success of their launch based on weekly script trends. The difference between social media data and data derived from prescriptions is significant: social media data can predict the future, while script data record the past. Social media can also, to some degree, explain events and not just record them, since patient posts are much more nuanced than purchase data, often sharing the why and not just the what. Using older methods, it can take years to understand the result and impact of a new drug launch. Today social media can provide early vital signals in real time. To illustrate this, let’s look at Tecfidera (formerly called BG-12 during clinical trials), a new multiple sclerosis drug that Biogen launched on April 13 [through an examination of the patient voice from billions of patient-written social media posts on over 2,000 health blogs and forums]. Interestingly, since the launch of Tecfidera in mid-April, the most talked about MS drug in social media has been Tecfidera, bypassing all other MS medications and growing on a daily basis. We also see significant differences between Tecfidera discussions and that of other MS medications in that 36% of the Tecfidera discussions are on Facebook while for other MS medications only 28% of the discussions are taking place on Facebook (our analysis does not include Twitter).

|
Scooped by
rob halkes
May 31, 2013 5:11 AM
|
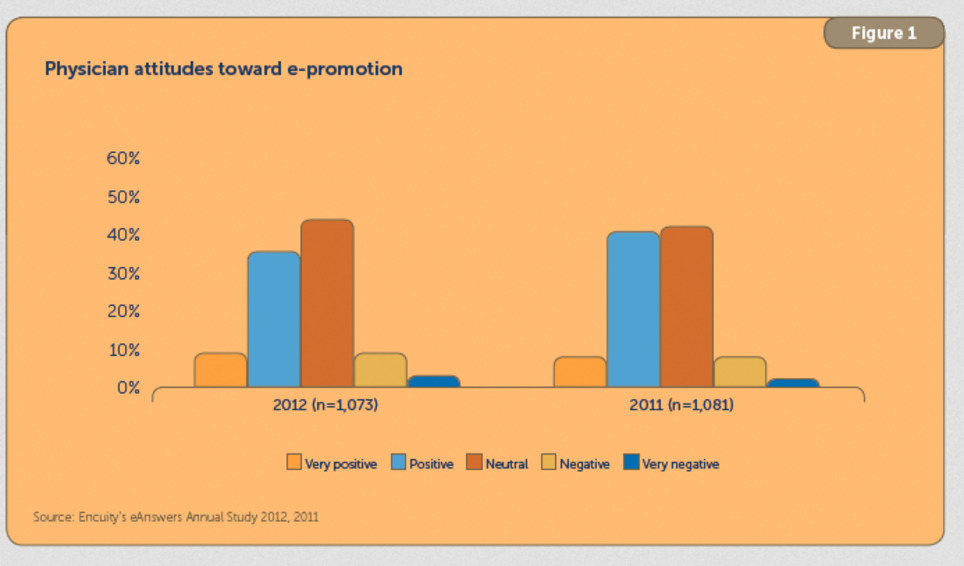
Based on a comScore longitudinal study of a permission-based panel of 1,000 U.S. physicians HCP Content websites such as Medscape.com, which provide content or services catering specifically to physicians, reached the highest percentage of physicians (81 percent) in comparison to other types of health sites. While there is a significant increase in eDetailing budgets the biggest challenge for drug companies is do physicians have the time to engage drug companies electronically ?

|
Scooped by
rob halkes
May 16, 2013 8:18 AM
|
The pharmaceutical industry's understanding and adoption of multichannel strategy is, at best, variable. While more enlightened organisations are trying hard to challenge the old models of sales and marketing, others are stubbornly sitting on their hands and persisting with tried-and-tested traditional methodology. ..... In truth, it's simply paper under glass. To progress, companies perhaps need to become more proactive and pull multichannel planning into the strategic process much earlier. But to do this, they may need to develop a greater understanding of the opportunity and challenge some of the historical cultural values that are deeply embedded across the industry. .. “Thankfully, we appear to be coming to the end of the industry-wide obsession with digital and reaching a more level playing field, where companies realise there's a balance to be struck. But to move forward, we really need to follow consumer marketing's lead and develop an empirical way of measuring the value of activity. That will significantly inform our ability to plan a multichannel approach.” .. At present, the industry's use of multichannel marketing is often tactical - and rather than being stitched into the fabric of a properly planned pre-launch strategy, it's bolted on at the end as a product of the 'must do something digital' philosophy.

|
Scooped by
rob halkes
May 15, 2013 5:46 AM
|
Following up the presentation of our project with a pharma company in the Netherlands on how Pharma can enhance its impact and reduce costs of marketing, we present our chalkboard sketch to construe a new commercial model for a pharmaceutical market approach: An approach that would guide the decision makers in the company to construe the right set of activities to focus on accounts with the best chances of success, engage with its decision makers on drug prescription effectively and provide added value to their medicines so as to help care providers to help their patients better. The model starts with the acknowledgement that there is not one right way for approaching all different markets to pharma with a standard approach. Preference schemes for drug selection, local protocols, reimbursement schemes, actual health care systems’ conditions, drug prices, different stakeholders and the very portfolio of the company, can only lead to the conclusion that differentiation should be the name of the game. (see here). The model needs to accommodate for these different conditions.

|
Scooped by
rob halkes
May 3, 2013 1:10 PM
|
Since 2005, we developed and implemented a strategy to create a new pharma market approach in the Netherlands. Since 2010 it proves to be effective to grow ROI. Here (1/2) we describe concisely the project. In a few days, we will present a chalkboard sketch of the approach (2/2), based upon our review of the very project and our activities to change the market approach and make it all effective. It hasn’t been a very structured process, as experimentation and development often is not, but the innovation worked and we could collect its benefits to the ROI of our client, a local affiliate of a large global pharmaceutical company. We are now using the decision and action tools that we developed in the course of the project, to create more adaptive pharma roles for other companies as well. ..

|
Scooped by
rob halkes
April 8, 2013 2:26 PM
|
PatientView's latest PatientView Plus reports In January 2013, PatientView published ‘The Corporate Reputation of Pharma—the Patient Perspective', based on the opinions of 600 patient groups worldwide. PatientView is now releasing four reports on the performance of major pharma companies in Germany, Italy, UK and USA. Surprisingly, the corporate reputation of pharma, and the rankings of each of the pharma companies, varies significantly between the four countries. In 2012, 40% of 600 respondent patient groups believed that the corporate reputation of pharma had declined worldwide. However, patient groups' attitude to pharma varies significantly across the four countries analysed. Pharma in the USA stands out as worst among the four countries: 63% of respondent US patient groups believe that the corporate reputation of the pharma industry declined in 201240% of respondent UK patient groups believe that the corporate reputation of the pharma industry declined in 201237% of respondent Italian patient groups believe that the corporate reputation of the pharma industry declined in 201233% of respondent German patient groups believe that the corporate reputation of the pharma industry declined in 2012 Top pharma performers for 2012 Companies ranking top for corporate reputation in the different countries: Germany: JanssenItaly: PfizerUK: PfizerUSA: Bayer

|
Scooped by
rob halkes
April 8, 2013 11:29 AM
|
The Dutch Health Care Reform 2006, a reflection Having witnessed the evolvement of the Dutch Health Care Reform (see Wikipedia), it is inspiring to review it to evaluate what processes and forces have initiated the development and formed its course. Based upon our studies, we construed this presentation (see blog)
Of course, as in many advanced health care systems, as it is probably so too in emerging countries’ systems of health care, “finances” are the dominant driver. But the power of longing for a good health and care for health makes the world turn around. Health care systems all represent a common and core value in life: the personal health condition. It is closely monitored at different parameters to see how its development effects the very human condition. In discussing possibilities for moving desired developments, we were reflecting on what processes or factors might be used to work along to stimulate innovation and change. If we could do so, we could change the system for the better and alongside with it, develop our businesses when they team up with the same intentions: better and more care for less money. We had a very stimulating discussion about these possibilities to pharma companies about teaming up with the innovating forces in health care systems: we suggest a few in the presentation. Of course, it is way different from that what has been comprehended with the term “Market Access”. It could rather be changed into “market -” or “innovation collaboration.”

|
Scooped by
rob halkes
April 5, 2013 1:53 PM
|
Digitas Health has announced the release of Effective Review and Approval of Digital Promotional Tactics, written by Dale Cooke, who is its VP/group director, regulatory review with Digitas Health. The Food and Drug Law Institute published the comprehensive primer. In addition to an approachable and optimistic view of FDA’s most recent guidance on social media use, the primer provides guiding principles for how pharmaceutical marketers can embrace digital, while ensuring compliance. “People need to understand how to effectively review promotional tactics,” Cooke told Med Ad News. “I have heard people at top companies proudly announce that they are responsible for developing company guidelines for using social media despite the fact that they don't have a Facebook account. Imagine someone trying to review a television commercial without having ever seen a television. They wouldn't know the importance of asking about things such as how fast the voiceover is delivered or whether the music will overwhelm the audio. But those are extremely important factors that determine whether the resulting promotional material is compliant. Exactly the same is true of all media. Reviewers need to understand, and preferably use those media to appreciate all of the factors that contribute to whether a message is compliant.”

|
Scooped by
rob halkes
April 5, 2013 1:38 PM
|
Patient groups are hailing a new era of transparency, but drug companies fear its effects When Guido Rasi took charge of Europe’s medicines’ regulator in 2011 he inherited an explosive dossier that is now poised to transform drug development. The furore started in 2007 when two Danish academics pushed the European Medicines Agency for greater transparency on 15 clinical trials. Their pursuit of greater openness set in motion a chain of events that stands to revolutionise the pharmaceutical industry in the months ahead. From the beginning of next year, the EMA will release all information about clinical studies submitted to it by organisations seeking authorisation for new treatments. As this deadline approaches, heated parliamentary and legal debates are raging to determine how far this new culture of open access should go. For advocates of change, including many patient groups and academic researchers, greater transparency in drug trials is essential to understand the full risks and benefits of medicines and reduce development costs. Critics argue that a cascade of publicly available data will threaten the confidentiality of patients taking part in trials and undermine the role of regulators. Many pharmaceutical companies are watching the disputes with trepidation, fearing that the publication of so much trial data could damage their business models by allowing competitors to access the expensive research they undertook to develop their drugs... High quality global journalism requires investment. Please share this article with others using the link below, do not cut & paste the article. See our Ts&Cs and Copyright Policy for more detail. Email ftsales.support@ft.com to buy additional rights. http://www.ft.com/cms/s/0/c8b024b0-9b82-11e2-a820-00144feabdc0.html#ixzz2PbyFz4pV

|
Scooped by
rob halkes
April 4, 2013 1:17 PM
|
Maybe the gap between consumer packaged good marketers and pharma marketers is finally closing. According to a new Accenture Report ”the sales and marketing models of today need to be reshaped in order to be successful in today’s “new normal. Reducing costs, mastering multichannel marketing and improving digital effectiveness are the top strategic priorities for pharmaceutical sales and marketing executives.” Accenture says, There is a customer engagement revolution in motion in the Life Sciences industry as it faces life in the “new normal” after the peak of the patent cliff1. The industry is in an era where targeting specific populations and improving patient outcomes is critical for specialty products and where reaching more customers in rapidly developing markets is paramount for growth. This revolution is requiring companies to rethink how they can reach patients, payers, providers and governments in both mature and emerging markets—at speed, at the right price and with the right information for each target audience. This is a significant change from the “feet on the street,” single message selling model that worked well for blockbuster drugs in mature healthcare markets. And I think I finally found out why agency people are reporting that pharma is spending more in digital marketing. According to the report The use of third-party service providers is a relatively common practice in the industry that will continue to increase. ... The real challenge is can third parties really provide the best analytics and digital marketing strategy as someone who actually works within the brand team/company? It has been my experience that too many pharma companies treat third parties as vendors and don’t always share every bit of important information. Even more important outside agencies need to understand the dynamics, including the corporate politics, within the organization. Employees who don’t understand digital marketing and want to hold onto more “glamorous” channels like TV may fight the shift in dollars to analytics and digital marketing. Still this is great news for pharma which has a reputation of coming late to the party.

|
Scooped by
rob halkes
March 15, 2013 11:18 AM
|
Wer nach der Einnahme eines Medikamentes eine Nebenwirkung spürte, ging zum Arzt oder Apotheker. Doch ob das Problem weitergegeben wurde, wussten die wenigsten. Das kann jetzt jeder selbst ändern.
Patienten können in Deutschland jetzt direkt mögliche Nebenwirkungen von Arzneimitteln an Behörden melden. Das Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM) und das Paul-Ehrlich-Institut (PEI) für Impfstoffe starteten die Testphase für ein Internetportal, um solche Berichte schneller und einfacher erfassen zu können. Dadurch sollen unbekannte Nebenwirkungen früher als bisher erkannt und – wenn nötig – Maßnahmen zur Risikominimierung eingeleitet werden. Über das neue Internetportal verbraucher-uaw.pei.de haben Patienten und Verbraucher nunmehr Zugang zu einem (relativ) einfachen Meldeformular. .."

|
Scooped by
rob halkes
March 15, 2013 5:24 AM
|
Pharma’s new commercial model is not easily created. We see Pharma companies to add all sorts of new programs to their company, like “Closed Loop Marketing”, “Sales Force Effectiveness”, “Key Account Management”, “Key Opinion Leader Management”, “Customer First”, or “Customer Excellence”, “Market Access management” etc. Surely all kinds of innovations to focus and approach promotion in a new way.
Also, Social Media, Websites and On line Communities are created to “get the word out..”
But for now, we want to focus on edetailing as a core aspect for triggering the road map to create more effect and collaboration with customers (physicians and other caregivers) to support patients’ therapies, such as compliance support. ...
See presentation

|
Scooped by
rob halkes
March 9, 2013 7:15 AM
|
FirstWord Pharma - Gain Access to the Information You Need Track the Companies,
Products, and Regulatory Areas of Most Interest to You

|
Scooped by
rob halkes
March 8, 2013 1:11 PM
|
Compilation of pharmaceutical trends and strategies 2013 from Cutting Edge Information. The report gives a quick overview of key areas a pharmaceutical industry has to tend to for its business.. Download link to the pdf of the report

|
Scooped by
rob halkes
March 8, 2013 12:58 PM
|
Warum Soziale Ärztenetzwerke für Ihre Marketingstrategie unerlässlich seien werden

|
Scooped by
rob halkes
March 4, 2013 10:32 AM
|
My colleague Ritesh Patel (@ritters90 for those of you who don’t currently follow him) is going to be giving his thoughts from an event chairman’s perspective in the near future, but when it’s fresh in my mind I thought I’d give a perspective from the floor. To me, the most important thinking fell into three categories: - Pharma is finally putting multichannel in its proper context - Social is getting serious - Gamification is gaining proper meaning ..
|


 Your new post is loading...
Your new post is loading...
















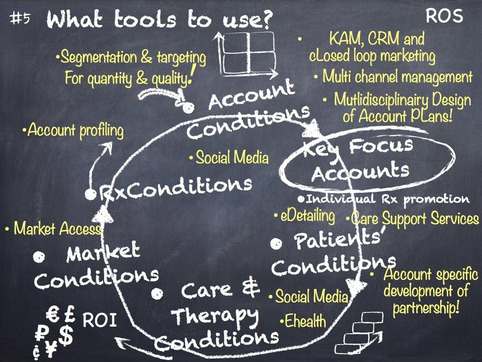













Great to see that the pharma branche is developing a new position as partner in health, not just as "a" commercial company.
It now needs to express this change in its commercial activities. Lots of opportunities there to redefine its market approach, e.g.
http://bit.ly/118Xk1a