Your new post is loading...
 Your new post is loading...

|
Scooped by
Gilbert C FAURE
October 17, 2022 11:45 AM
|
This topic is devoted to Neuro-immunology, covering various neurological disorders with confirmed or possible immunological mechanisms. This year (2022), many students are choosing Neurology and neurological diseases for their Scoop.it exercise. It will stimulate me for improving this topic (tagging...) to help them navigate the curated resources. Some key points: We developed original research on Choroid Plexuses in ageing and Alzheimer's disease. https://www.scoop.it/topic/neuroimmunology?q=choroid+plexuses The topic of autoimmunity at large, became very fashionable recently. https://www.scoop.it/topic/neuroimmunology?q=autoimmunity with prescription of many biological tests for diagnosis of difficult neurological clinical disorders. We were also very much involved, 15 years ago in the development of CSFTCs detection, enumeration and analysis in carcinoma meningitis, a rare but dramatic issue in breast, lung ... cancers and melanomas. Papers on https://www.scoop.it/topic/nancytomique

|
Scooped by
Gilbert C FAURE
October 8, 4:01 AM
|

|
Scooped by
Gilbert C FAURE
August 1, 7:02 AM
|
When people viewed virtual avatars with coughs or rashes, their brains triggered an immune response.

|
Scooped by
Gilbert C FAURE
July 24, 10:50 AM
|

|
Scooped by
Gilbert C FAURE
July 17, 8:11 AM
|
Connection and communication between the nervous and immune systems
- Connections between the nervous and immune systems are increasingly recognized as central to brain–body physiology. Rather than operating in isolation, the nervous and immune systems form an integrated network that is more than the sum of its parts.
- The nervous and immune systems perceive and process distinct yet overlapping stimuli from the external and internal environment. The nervous system primarily detects physical cues, including light, sound and touch, whereas the immune system specializes in molecular recognition of pathogens, antigens, cytokines and endogenous damage signals.
- Through bidirectional communication, immune-derived signals modulate neuronal activity, influencing pain, inflammation and sickness behaviours, whereas neural circuits regulate immune cell development and function. Together, these systems act together to orchestrate homeostasis and responses to stress and disease.
- Our understanding of neuroimmune interactions is still in its early stages, but their significance across various diseases is becoming increasingly evident. As research advances, these interactions may offer new therapeutic opportunities.
- For example, inhibiting immune cell trafficking to the brain is already a cornerstone of multiple sclerosis treatment and could soon be extended to psychiatric disorders such as treatment-resistant depression, where abnormal monocyte infiltration into the brain has a role.
https://lnkd.in/eAfce3R7
#immunology #immunesystem #science #neuroimmunology

|
Scooped by
Gilbert C FAURE
July 7, 10:24 AM
|
Advancements in B-cell therapies, including anti-CD20 and anti-CD19 monoclonal antibodies, enhance treatment efficacy and safety for multiple sclerosis and autoimmune diseases.

|
Scooped by
Gilbert C FAURE
June 13, 6:29 AM
|

|
Scooped by
Gilbert C FAURE
May 7, 10:14 AM
|

|
Scooped by
Gilbert C FAURE
April 23, 7:20 AM
|

|
Scooped by
Gilbert C FAURE
March 21, 5:20 AM
|
🧠🧬 Focus sur les cellules CAR-T en neurologie, ainsi que sur des dossiers transversaux et la pharmacologie. Encore un numéro incontournable de 𝙇𝙖…

|
Scooped by
Gilbert C FAURE
February 25, 4:18 AM
|
New review from our lab published in BRAIN
We explore how molecular blood-brain barrier (BBB) changes in aging, neurodegeneration and stroke affect disease…

|
Scooped by
Gilbert C FAURE
February 13, 6:48 AM
|
There is a misconception that only amyloid-targeting treatments have been studied in Alzheimer's disease. The reality is that clinical trials have evaluated… | 21 comments on LinkedIn

|
Scooped by
Gilbert C FAURE
February 10, 2:43 AM
|
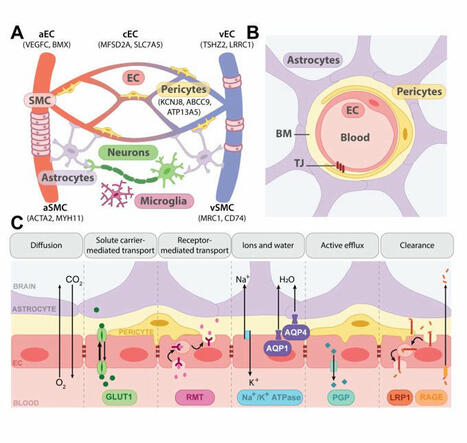
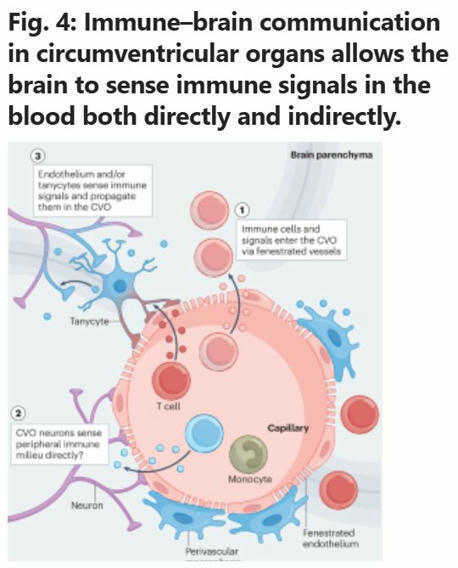
Immune control of brain physiology
Contrary to the classical view of immune-brain communication as a dormant system activated only during pathology, this…
|

|
Scooped by
Gilbert C FAURE
Today, 2:03 PM
|

|
Scooped by
Gilbert C FAURE
August 7, 3:25 AM
|
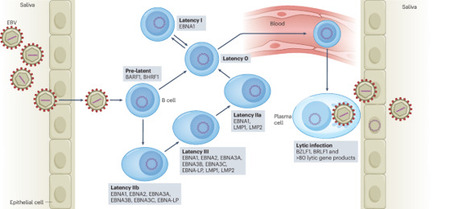
Epidemiological data have identified Epstein–Barr virus (EBV) infection as the main environmental risk factor for multiple sclerosis, the predominant autoimmune disease of the central nervous system (CNS)1. However, how EBV infection initiates multiple sclerosis pathogenesis remains unclear. Here we demonstrate that EBV expands oligoclonal T-bet+CXCR3+ B cells that home to the CNS in humanized mice. Effector memory CD8+ T cells and CD4+ TH1 cells as well as CD4+ TH17 cells co-migrate to the brain of EBV-infected humanized mice. T-bet+CXCR3+ B cells can colonize submeningeal brain regions in the absence of other lymphocytes and attract T cells. Depletion of B cells with rituximab or blocking of CXCR3 significantly decreases lymphocyte infiltration into the CNS. Thus, we suggest that symptomatic primary EBV infection generates B cell subsets that gain access to the CNS, attract T cells and thereby initiate multiple sclerosis. Epstein–Barr virus infection generates a neuroinvasive B cell subset, which recruits activated T cells to the central nervous system, promoting multiple sclerosis.

|
Scooped by
Gilbert C FAURE
July 29, 2:58 AM
|
Understanding the Neurologic Impact of Influenza and the Protective Role of Vaccination 🫁 🧠
Influenza is widely recognized as a respiratory infection affecting people across all age groups, leading to substantial illness and death. While respiratory complications are well known, the nonpulmonary effects—particularly neurologic complications—are often overlooked.
Neurologic issues such as seizures, encephalitis, myelitis, and Guillain-Barré Syndrome can occur during acute influenza infections, though these are relatively rare. More concerning, however, is emerging evidence linking influenza with long-term risks like stroke and dementia—two of the leading causes of death and disability worldwide.
💉 Encouragingly, recent studies suggest that influenza vaccination may offer protective benefits beyond preventing flu itself, potentially reducing the risk of stroke and dementia.
This highlights the importance of flu vaccination not only for respiratory health but also for protecting our brain health.
https://lnkd.in/emQnFrHd
#Influenza #Neurology #PublicHealth #Vaccination #StrokePrevention #DementiaAwareness #Healthcare

|
Scooped by
Gilbert C FAURE
July 22, 3:56 AM
|
While caring for a patient with pancreatic cancer during medical school, HMS alum William Hwang was surprised to learn that malignant cells can surround and invade nerves — a painful phenomenon known as perineurial invasion.
“I wondered why cancer cells would form such intimate connections with nerves,” he recalls. “What do they derive from this relationship, and how can we intervene?”
Now an HMS professor of radiation oncology at Massachusetts General Hospital, Hwang is helping to pioneer a new field known as cancer neuroscience. His lab uses spatial transcriptomics — an approach that combines advanced imaging with RNA sequencing and complex algorithms analyzing massive data sets — to pinpoint the genes expressed within individual cells in different areas of a tumor. These new techniques help his team identify differences between tumor cells that are engaged with nerves and those that aren’t.
Growing cancer cells together with nerves in dishes, Hwang’s team recently identified molecules produced by cancer cells that encourage nerves to grow toward and invade tumors. They’re now figuring out how to intervene in this process to prevent cancer cells from invading nerves, ultimately improving outcomes for patients. | 11 comments on LinkedIn

|
Scooped by
Gilbert C FAURE
July 17, 8:07 AM
|

|
Scooped by
Gilbert C FAURE
June 25, 9:14 AM
|
Sixty years after its discovery as the first human tumour virus, Epstein–Barr virus (EBV)-specific therapies and vaccines have entered clinical trials. These might not only be applicable for EBV-associated malignancies, where the virus was originally discovered, but also to immunopathologies, including the autoimmune disease multiple sclerosis, which might be triggered in susceptible individuals by primary EBV infection. This Review discusses the surprisingly large spectrum of diseases that EBV seems to cause, as well as which of these might be treated by the therapeutic approaches that are currently being developed or are already clinically applied. New pharmacological inhibitors, antibody therapies, adoptive T cell therapies and active vaccinations are beginning to offer possibilities to target the various EBV infection programmes that are associated with different diseases. These novel developments might allow us to specifically target EBV rather than its host cells in virus-associated pathologies. Sixty years after the discovery of the Epstein–Barr virus (EBV), Münz discusses new developments in the immunobiology of the first discovered human tumour virus, including vaccines and the role of EBV in the initiation of autoimmune diseases, especially multiple sclerosis.

|
Scooped by
Gilbert C FAURE
May 15, 1:38 PM
|
Independent of antigen presentation, migratory CCR7+ dendritic cells orchestrate the influx, proliferation and cytotoxic action of natural killer cells to control cancer cell growth in the leptomeninges.

|
Scooped by
Gilbert C FAURE
May 5, 4:11 AM
|

|
Scooped by
Gilbert C FAURE
March 28, 4:51 AM
|
Les ressources de notre cerveau sont exceptionnelles et souvent méconnues. Par exemple, il continue à générer de nouveaux neurones, même à l’âge adulte. Il… | 26 comments on LinkedIn

|
Scooped by
Gilbert C FAURE
March 3, 9:51 AM
|
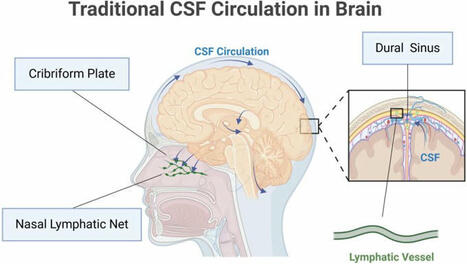
🧠 Glymphatic system: a self-purification circulation in brain🌊💡
The glymphatic system offers a fresh perspective on fluid dynamics and homeostasis in the… | 13 comments on LinkedIn

|
Scooped by
Gilbert C FAURE
February 22, 8:54 AM
|

|
Scooped by
Gilbert C FAURE
February 10, 4:15 AM
|
𝐂𝐞𝐥𝐥 𝐓𝐡𝐞𝐫𝐚𝐩𝐲 𝐢𝐧 𝐍𝐞𝐮𝐫𝐨𝐬𝐜𝐢𝐞𝐧𝐜𝐞 𝐋𝐚𝐧𝐝𝐬𝐜𝐚𝐩𝐞 🧠
With 50+ companies tackling 600+ neurological disorders, the opportunity to impact… | 17 comments on LinkedIn

|
Scooped by
Gilbert C FAURE
February 9, 4:15 AM
|
|



 Your new post is loading...
Your new post is loading...