Your new post is loading...
 Your new post is loading...

|
Rescooped by
Gilbert C FAURE
from Virus World
November 6, 2023 3:40 AM
|
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA generally becomes undetectable in upper airways after a few days or weeks postinfection. Here we used a model of viral infection in macaques to address whether SARS-CoV-2 persists in the body and which mechanisms regulate its persistence. Replication-competent virus was detected in bronchioalveolar lavage (BAL) macrophages beyond 6 months postinfection. Viral propagation in BAL macrophages occurred from cell to cell and was inhibited by interferon-γ (IFN-γ). IFN-γ production was strongest in BAL NKG2r+CD8+ T cells and NKG2Alo natural killer (NK) cells and was further increased in NKG2Alo NK cells after spike protein stimulation. However, IFN-γ production was impaired in NK cells from macaques with persisting virus. Moreover, IFN-γ also enhanced the expression of major histocompatibility complex (MHC)-E on BAL macrophages, possibly inhibiting NK cell-mediated killing. Macaques with less persisting virus mounted adaptive NK cells that escaped the MHC-E-dependent inhibition. Our findings reveal an interplay between NK cells and macrophages that regulated SARS-CoV-2 persistence in macrophages and was mediated by IFN-γ. Huot et al. show that interferon-γ (IFN-γ) regulates the persistence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in bronchoalveolar macrophages from cynomolgus macaques up to 18 months postinfection. Published in Nat. Immunology (Nov. 2, 2023): https://doi.org/10.1038/s41590-023-01661-4
Via Juan Lama

|
Scooped by
Gilbert C FAURE
June 24, 2023 2:58 AM
|
After having successfully tested a mucosal vaccine against coronavirus in animals just months ago, Berlin scientists have now further developed their live SARS-CoV-2 vaccine. Researchers at Freie Universität Berlin have increased the safety of the vaccine, which is administered via the nose. The results of their study on the modified form of the live vaccine have been published in Molecular Therapy.

|
Scooped by
Gilbert C FAURE
November 27, 2022 3:37 AM
|
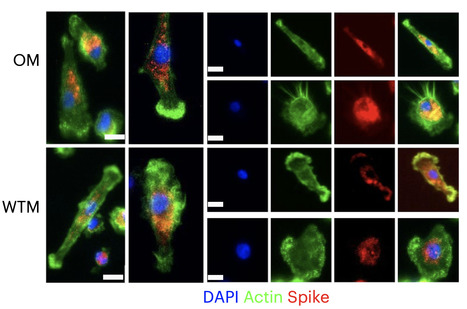
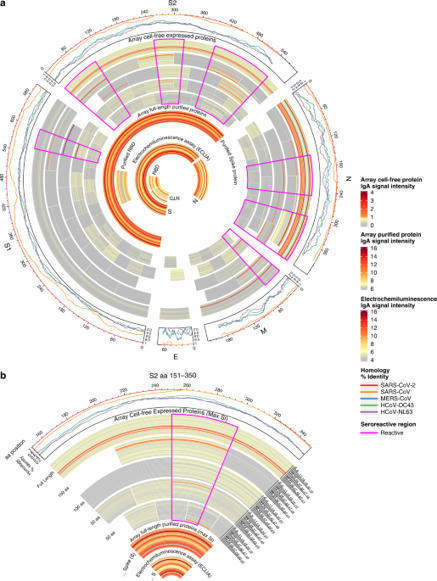
One potential mechanism for protection from SARS-CoV-2 in children is through passive immunity via breast milk from a mother infected with the novel coronavirus. The primary objectives of this study were to establish the presence of SARS-CoV-2-specific IgA and IgG and to characterize the antigenic regions of SARS-CoV-2 proteins that were reactive with antibodies in breast milk. Between March 2020 and September 2020, 21 women with confirmed SARS-CoV-2 infection were enrolled in Mommy’s Milk. Participants donated serial breast milk samples around their time of illness. Breast milk samples were used to probe a multi-coronavirus protein microarray containing full-length and variable-length overlapping fragments of SARS-CoV-2 proteins. Samples were also tested against S and N proteins by electrochemiluminescence assay. The breast milk samples contained IgA reactive with a variety of SARS-CoV-2 antigens. The most IgA-reactive SARS-CoV-2 proteins were N (42.9% of women responded to ≥1 N fragment) and S proteins (23.9% responded to ≥1 fragment of S1 or S2). IgG responses were similar. A striking observation was the dissimilarity between mothers in antibody recognition, giving distinct antibody reactivity and kinetic profiles. Individual COVID-19 cases had diverse and unique milk IgA profiles following the onset of symptoms.

|
Scooped by
Gilbert C FAURE
November 15, 2022 4:56 AM
|
The SARS-CoV-2 pandemic provides a natural opportunity for the collision of coronavirus disease-2019 (COVID-19) with chronic infections, which place numerous individuals at high risk of severe COVID-19.

|
Scooped by
Gilbert C FAURE
August 18, 2022 5:11 AM
|
Analysis: a bivalent vaccine has been approved and research is being carried out into possible pan-coronavirus vaccines...

|
Scooped by
Gilbert C FAURE
May 12, 2022 11:23 AM
|
Scientists are studying whether long COVID could be linked to viral fragments found in the body months after initial infection

|
Scooped by
Gilbert C FAURE
December 30, 2021 4:17 AM
|
BackgroundLimited data are available regarding the balance of risks and benefits from human milk and/or breastfeeding during and following maternal infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).ObjectiveTo investigate whether SARS-CoV-2 can be detected in milk and on...

|
Scooped by
Gilbert C FAURE
November 9, 2021 5:25 AM
|
Immunity to pulmonary infection typically requires elicitation of lung-resident T cells that subsequently confer protection against secondary infection. The presence of tissue-resident T cells in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) convalescent patients is unknown. Using a sublethal mouse model of coronavirus disease 2019, we determined if SARS-CoV-2 infection potentiated Ag-specific pulmonary resident CD4+ and CD8+ T cell responses and if these cells mediated protection against secondary infection. S protein–specific T cells were present in resident and circulating populations. However, M and N protein–specific T cells were detected only in the resident T cell pool. Using an adoptive transfer strategy, we found that T cells from SARS-CoV-2 immune animals did not protect naive mice. These data indicate that resident T cells are elicited by SARS-CoV-2 infection but are not sufficient for protective immunity.

|
Scooped by
Gilbert C FAURE
September 14, 2021 5:14 AM
|
Despite remarkable progress in the development and authorization of vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the…...

|
Scooped by
Gilbert C FAURE
August 4, 2021 2:08 PM
|
Scientists from Germany and Italy recently demonstrated the benefits of intramuscular/intranasal heterologous prime-boost vaccination regimen against coronavirus disease 2019 (COVID-19).

|
Scooped by
Gilbert C FAURE
August 2, 2021 3:56 AM
|
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to scale up around the world, costing severe health and economic losses.

|
Scooped by
Gilbert C FAURE
June 4, 2021 1:41 PM
|
Resistance represents a major challenge for antibody-based therapy for coronavirus disease 2019 (COVID-19)1–4. Here we engineered an immunoglobulin M (IgM) neutralizing antibody (IgM-14) to overcome the resistance encountered by IgG-based therapeutics. IgM-14 is >230-fold more potent than its parental IgG-14 in neutralizing the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). IgM-14 potently neutralizes the resistant virus raised by its corresponding IgG-14, the newly emerged United Kingdom B.1.1.7, Brazilian P.1, and South African B.1.351 variants of concern (VOCs), and 21 other receptor-binding domain (RBD) mutants, many of which are resistant to the IgGs that have been authorized for emergency use. Although engineering IgG into IgM enhances antibody potency in general, selection of an optimal epitope is critical for identifying the most effective IgM that can overcome resistance. One single intranasal (IN) dose of 0.044 and 0.4 mg/kg IgM-14 confers prophylactic and therapeutic efficacy against SARS-CoV-2 in mice, respectively. IgM-14, but not IgG-14, also confers potent therapeutic protection against the P.1 and B.1.351 variants. IgM-14 exhibits desirable IN pharmacokinetics and safety in rodents. Our results demonstrate that IN administration of an engineered IgM can improve efficacy, reduce resistance, and simplify the prophylactic and therapeutic treatment of COVID-19.

|
Scooped by
Gilbert C FAURE
May 17, 2021 3:41 AM
|
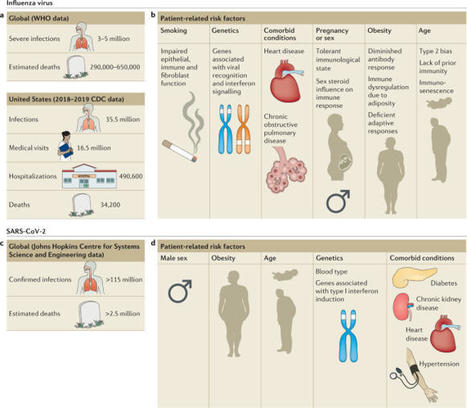
Influenza viruses cause annual epidemics and occasional pandemics of respiratory tract infections that produce a wide spectrum of clinical disease severity in humans. The novel betacoronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in December 2019 and has since caused a pandemic. Both viral and host factors determine the extent and severity of virus-induced lung damage. The host’s response to viral infection is necessary for viral clearance but may be deleterious and contribute to severe disease phenotypes. Similarly, tissue repair mechanisms are required for recovery from infection across the spectrum of disease severity; however, dysregulated repair responses may lead to chronic lung dysfunction. Understanding of the mechanisms of immunopathology and tissue repair following viral lower respiratory tract infection may broaden treatment options. In this Review, we discuss the pathogenesis, the contribution of the host response to severe clinical phenotypes and highlight early and late epithelial repair mechanisms following influenza virus infection, each of which has been well characterized. Although we are still learning about SARS-CoV-2 and its disease manifestations in humans, throughout the Review we discuss what is known about SARS-CoV-2 in the context of this broad knowledge of influenza virus, highlighting the similarities and differences between the respiratory viruses. In this Review, Schultz-Cherry, Thomas and colleagues discuss the pathogenesis of influenza virus and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the human respiratory tract, the contribution of the host response to severe disease, epithelial repair mechanisms following infection, and current and potential future therapies for influenza virus and SARS-CoV-2 infections.
|

|
Scooped by
Gilbert C FAURE
July 22, 2023 5:45 AM
|
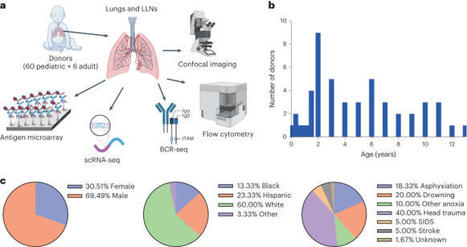
Infants and young children are more susceptible to common respiratory pathogens than adults but can fare better against novel pathogens like severe acute respiratory syndrome coronavirus 2. The mechanisms by which infants and young children mount effective immune responses to respiratory pathogens are unknown. Through investigation of lungs and lung-associated lymph nodes from infant and pediatric organ donors aged 0–13 years, we show that bronchus-associated lymphoid tissue (BALT), containing B cell follicles, CD4+ T cells and functionally active germinal centers, develop during infancy. BALT structures are prevalent around lung airways during the first 3 years of life, and their numbers decline through childhood coincident with the accumulation of memory T cells. Single-cell profiling and repertoire analysis reveals that early life lung B cells undergo differentiation, somatic hypermutation and immunoglobulin class switching and exhibit a more activated profile than lymph node B cells. Moreover, B cells in the lung and lung-associated lymph nodes generate biased antibody responses to multiple respiratory pathogens compared to circulating antibodies, which are mostly specific for vaccine antigens in the early years of life. Together, our findings provide evidence for BALT as an early life adaptation for mobilizing localized immune protection to the diverse respiratory challenges during this formative life stage. Young children frequently encounter respiratory pathogens that elicit immune responses in developing lungs. Farber and colleagues examine rare lung tissue samples obtained from pediatric organ donors and find age-dependent formation of bronchus-associated lymphoid tissue (BALT), which peaks at 3 years of age and dissipates thereafter. Profiling of BALT lymphocytes indicates that repertoire and functional differences exist between the lung, draining lymph nodes and circulating cells.

|
Scooped by
Gilbert C FAURE
April 1, 2023 12:07 PM
|
Protective immune responses against respiratory pathogens, such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and influenza virus, are initiated by the mucosal immune system.However, most licensed vaccines are administered parenterally and are largely ineffective at inducing mucos...

|
Scooped by
Gilbert C FAURE
November 15, 2022 11:38 AM
|
Coronavirus Disease-19 (COVID-19) symptoms range from mild to severe illness; the cause for this differential response to infection remains unknown. Unravelling the immune mechanisms acting at different levels of the colonization process might be key ...

|
Rescooped by
Gilbert C FAURE
from Virus World
August 18, 2022 2:04 PM
|
One ‘superspreader’ with Omicron shed three times as much viral RNA as those with Alpha or Delta. People infected with the highly transmissible Alpha, Delta and Omicron variants of SARS-CoV-2 spew out higher amounts of virus than do those infected with other variants, according to a study1. Furthermore, individuals who contract COVID-19 after vaccination, and even after a booster dose, still shed virus into the air. The work was posted on the medRxiv preprint server on 29 July. It has not yet been peer reviewed. “This research showed that all three of those variants that have won the infection race … come out of the body more efficiently when people talk or shout than the earliest strains of the coronavirus,” says John Volckens, a public-health engineer at Colorado State University in Fort Collins. Study co-author Kristen Coleman, who researches emerging infectious diseases at the University of Maryland in College Park, says this means that people should be “pushing governments to invest in improving indoor air quality by improving ventilation and filtration systems”. Breathe out For the study, Coleman and her colleagues recruited 93 people between mid-2020 and early 2022 who were infected with SARS-CoV-2. Participants’ infections were caused by strains including the Alpha variant, which emerged in late 2020, and the later Delta and Omicron variants. All participants with the latter two strains had been fully vaccinated before catching the virus. The infected people faced into a cone-shaped apparatus and sang and shouted — with inevitable coughs and sneezes in between — for 30 minutes, while an attached machine collected the particles they exhaled. The device, called a Gesundheit-II, separated out the fine ‘aerosol’ droplets measuring 5 micrometres or less in diameter, which can linger in the air and leak through cloth and surgical masks. The team found that participants infected with the Alpha, Delta and Omicron variants emitted significantly more viral RNA when exhaling than did people infected with other variants. These include ancestral variants, such as the one first detected in Wuhan, China, and those not associated with increased transmissibility — such as Gamma, which arose in late 2020. For participants with Delta and Omicron, their fine aerosol contained on average five times the amount of virus that was detected in their larger, coarse aerosol. The team also seeded cells in the laboratory with aerosol samples and found that four samples, each from a participant with either Delta or Omicron, infected the cells. Shed virus is not always infectious, says study co-author Jianyu Lai, an epidemiologist at the University of Maryland, and the samples’ ability to infect laboratory cells means that viral RNA in exhaled aerosols can spread the disease. Malin Alsved, an aerosol technology scientist at Lund University in Sweden, says: “I’m bit concerned that they mix all the respiratory [aerosols] — they have breathing, talking, speaking, screaming, coughing, even sneezing in the sample.” Coleman responds that the team combined respiratory samples to mimic a real-life scenario such as being in a restaurant. Going viral The study also highlights variation between individuals in the amounts of exhaled virus, which ranged from non-detectable levels to those associated with ‘superspreaders’. One Omicron-infected participant, for example, shed 1,000 times as much viral RNA through fine aerosol as the maximum level observed in those with Alpha or Delta. The researchers say that the root of these discrepancies remains a mystery but could be related to biological factors such as a person’s age. Behaviour might play a part, too: the study’s superspreader coughed more frequently than others. If new variants are more prone to superspreading, that might drive them to dominate COVID-19 cases. The team notes that people infected with SARS-CoV-2 exhale much lower amounts of viral RNA than do people infected with influenza, a comparable airborne disease. This suggests that SARS-CoV-2 could spin off variants that transmit even more virus. “That is something to be concerned about,” says Alsved. Published in Nature (August 17, 2022): https://doi.org/10.1038/d41586-022-02202-z
Via Juan Lama

|
Scooped by
Gilbert C FAURE
May 23, 2022 11:03 AM
|
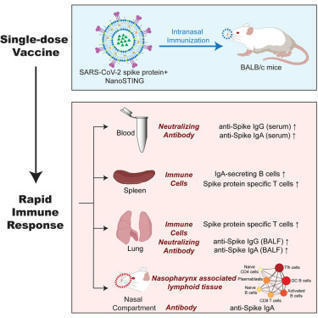
Mucosal vaccines offer the potential to trigger robust protective immune responses at the predominant sites of pathogen infection. In principle, the induction of adaptive immunity at mucosal sites, involving secretory antibody responses and tissue-resident T cells, has the capacity to prevent an infection from becoming established in the first place, rather than only curtailing infection and protecting against the development of disease symptoms. Although numerous effective mucosal vaccines are in use, the major advances seen with injectable vaccines (including adjuvanted subunit antigens, RNA and DNA vaccines) have not yet been translated into licensed mucosal vaccines, which currently comprise solely live attenuated and inactivated whole-cell preparations. The identification of safe and effective mucosal adjuvants allied to innovative antigen discovery and delivery strategies is key to advancing mucosal vaccines. Significant progress has been made in resolving the mechanisms that regulate innate and adaptive mucosal immunity and in understanding the crosstalk between mucosal sites, and this provides valuable pointers to inform mucosal adjuvant design. In particular, increased knowledge on mucosal antigen-presenting cells, innate lymphoid cell populations and resident memory cells at mucosal sites highlights attractive targets for vaccine design. Exploiting these insights will allow new vaccine technologies to be leveraged to facilitate rational mucosal vaccine design for pathogens including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and for cancer. Here, Ed Lavelle and Ross Ward discuss the unique aspects of mucosal immunity that must be considered when developing effective mucosal vaccines. The authors highlight the key immune cell populations that are targeted by mucosal vaccination strategies and explain how innovative adjuvant and delivery approaches should lead to new vaccines for infectious diseases and cancers.

|
Scooped by
Gilbert C FAURE
February 19, 2022 2:15 AM
|
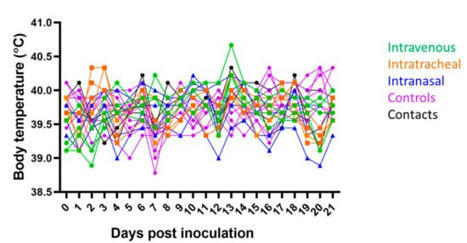
Since the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the susceptibility of animals and their potential to act as reservoirs or intermediate hosts for the virus has been of significant interest. Pigs are susceptible to multiple coronaviruses and have been used as an animal model for other human infectious diseases. Research groups have experimentally challenged swine with human SARS-CoV-2 isolates with results suggesting limited to no viral replication. For this study, a SARS-CoV-2 isolate obtained from a tiger which is identical to human SARS-CoV-2 isolates detected in New York City and contains the D614G S mutation was utilized for inoculation. Pigs were challenged via intravenous, intratracheal, or intranasal routes of inoculation (n = 4/route). No pigs developed clinical signs, but at least one pig in each group had one or more PCR positive nasal/oral swabs or rectal swabs after inoculation. All pigs in the intravenous group developed a transient neutralizing antibody titer, but only three other challenged pigs developed titers greater than 1:8. No gross or histologic changes were observed in tissue samples collected at necropsy. In addition, no PCR positive samples were positive by virus isolation. Inoculated animals were unable to transmit virus to naïve contact animals. The data from this experiment as well as from other laboratories supports that swine are not likely to play a role in the epidemiology and spread of SARS-CoV-2.

|
Scooped by
Gilbert C FAURE
December 19, 2021 9:48 AM
|
The lungs are the primary target of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, with severe hypoxia being the cause of death in the most critical cases. Coronavirus disease 2019 (COVID-19) is extremely heterogeneous in terms of severity, clinical phenotype and, importantly, global distribution. Although the majority of affected patients recover from the acute infection, many continue to suffer from late sequelae affecting various organs, including the lungs. The role of the pulmonary vascular system during the acute and chronic stages of COVID-19 has not been adequately studied. A thorough understanding of the origins and dynamic behaviour of the SARS-CoV-2 virus and the potential causes of heterogeneity in COVID-19 is essential for anticipating and treating the disease, in both the acute and the chronic stages, including the development of chronic pulmonary hypertension. Both COVID-19 and chronic pulmonary hypertension have assumed global dimensions, with potential complex interactions. In this Review, we present an update on the origins and behaviour of the SARS-CoV-2 virus and discuss the potential causes of the heterogeneity of COVID-19. In addition, we summarize the pathobiology of COVID-19, with an emphasis on the role of the pulmonary vasculature, both in the acute stage and in terms of the potential for developing chronic pulmonary hypertension. We hope that the information presented in this Review will help in the development of strategies for the prevention and treatment of the continuing COVID-19 pandemic. In this Review, the authors discuss the potential causes of the heterogeneity of COVID-19 and summarize the pathobiology of the disease, with an emphasis on the role of the pulmonary vasculature in the acute stage and the potential for developing chronic pulmonary hypertension.

|
Scooped by
Gilbert C FAURE
September 24, 2021 2:23 PM
|
The coronavirus disease 2019 (COVID-19) pandemic has highlighted the urgent need for effective prophylactic vaccination to prevent the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).

|
Scooped by
Gilbert C FAURE
August 7, 2021 12:04 PM
|
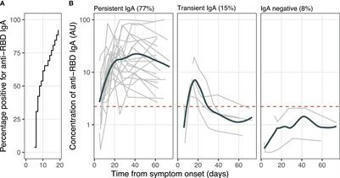
The outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a global concern. Immunoglobin A (IgA) contributes to virus neutralization at the early stage of infection. Longitudinal studies are needed to assess whether SARS-CoV-2-specific IgA production persists for a...

|
Scooped by
Gilbert C FAURE
August 2, 2021 3:56 AM
|
Amid concerns over a possible third wave of the coronavirus pandemic and to speed up the vaccination process in the country, an expert panel of India's central drug authority has recommended giving an approval to Bharat Biotech to carry out a study on interchangeability of its Covaxin and the...

|
Scooped by
Gilbert C FAURE
July 31, 2021 12:43 PM
|
Hassan et al. show that immunization with ChAd-SARS-CoV-2-S is durably immunogenic
and protects against SARS-CoV-2 challenge in a dose-dependent manner. Many months
after single-dose intranasal immunization, ChAd-SARS-CoV-2 confers protection against
variants of concerns of SARS-CoV-2 in both the upper and lower respiratory tracts
of mice.

|
Scooped by
Gilbert C FAURE
May 29, 2021 4:38 AM
|
Conventional smoking is known to both increase susceptibility to infection and drive inflammation within the lungs. Recently, smokers have been found to be at higher risk of developing severe forms of coronavirus disease 2019 (COVID-19).
|



 Your new post is loading...
Your new post is loading...