Your new post is loading...
 Your new post is loading...

|
Scooped by
Gilbert C FAURE
March 6, 2021 4:32 AM
|
medRxiv - The Preprint Server for Health Sciences

|
Scooped by
Gilbert C FAURE
February 21, 2021 4:17 AM
|
Preclinical tests at UAB last year showed potent systemic and mucosal immune responses in mice after a single intranasal dose. The vaccine candidate was developed by Maryland-based Altimmune Inc. Credit: UAB BIRMINGHAM, Ala.

|
Suggested by
LIGHTING
February 16, 2021 3:51 AM
|

|
Scooped by
Gilbert C FAURE
January 18, 2021 1:26 PM
|
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has infected 78 million individuals and is responsible for over 1.7 million deaths to date. Infection is associated with development of variable levels of antibodies with neutralizing activity that can protect against infection in animal models1,2. Antibody levels decrease with time, but the nature and quality of the memory B cells that would be called upon to produce antibodies upon re-infection has not been examined. Here we report on the humoral memory response in a cohort of 87 individuals assessed at 1.3 and 6.2 months after infection. We find that IgM, and IgG anti-SARS-CoV-2 spike protein receptor binding domain (RBD) antibody titres decrease significantly with IgA being less affected. Concurrently, neutralizing activity in plasma decreases by fivefold in pseudotype virus assays. In contrast, the number of RBD-specific memory B cells is unchanged. Memory B cells display clonal turnover after 6.2 months, and the antibodies they express have greater somatic hypermutation, increased potency and resistance to RBD mutations, indicative of continued evolution of the humoral response. Analysis of intestinal biopsies obtained from asymptomatic individuals 4 months after the onset of coronavirus disease-2019 (COVID-19), using immunofluorescence, or polymerase chain reaction, revealed persistence of SARS-CoV-2 nucleic acids and immunoreactivity in the small bowel of 7 out of 14 volunteers. We conclude that the memory B cell response to SARS-CoV-2 evolves between 1.3 and 6.2 months after infection in a manner that is consistent with antigen persistence.

|
Scooped by
Gilbert C FAURE
January 14, 2021 2:27 PM
|
bioRxiv - the preprint server for biology, operated by Cold Spring Harbor Laboratory, a research and educational institution

|
Scooped by
Gilbert C FAURE
January 12, 2021 11:00 AM
|

|
Scooped by
Gilbert C FAURE
January 11, 2021 5:14 AM
|
Mucosal Immunity: Feces and Covid-19
Mucosal Immunity : Feces and Covid-19 Surprisingly, although detection of virus in feces has been reported since the beginning of pandemy in China, mainly in children, the number of published papers focusing on this topic remain very low (# 250 papers in one year of pandemy, i.e 0,5% of Sars-Cov-2 published papers, more than 55000 according to PuBMed). Applications in diagnosis, epidemiology and follow-up of individual patients are almost inexistent. However, detection of viral material in effluents raised interest in published papers and are regularly reported in media. This short opinion paper wants to address this issue and stimulate researchers and diagnostic companies developping clinical research projects in this direction. If you consider results from search in PubMed , most papers (46%) originate from China some papers (10%) relate to animal coronavirus infection, and at least 15% report on sewage analysis. Papers reporting data on human diagnostic detection of virus in feces represent at most 50% of this collection. Clinically, the gastrointestinal tract is affected by COVID-19, gastrointestinal symptoms ranging from 7 to 60%, including diarrhea, nausea/vomiting, anorexia and abdominal pain. Biologically, presence of viral RNA in stools is rarely tested, but positive up to 50%. Virus was detected later than in sputum and respiratory secretions up to several weeks after clinical symptoms. RNA material is detected with frequently dynamic intra-host variations during convalescence, and sometimes probably viable virus potentially infectious. In the context, some chinese authors recommend including feces analysis particularly for discharge criteria Epidemiological and environment data were established through detection of virus RNA in sewage and wastewaters and also in solid fraction. These tests appear efficient for detecting new waves of cases and are apparently widely in use in Europe. Risk of transmission should be higher in developing countries where sanitation remains poor but data are lacking from such countries. During the past few weeks, China has been also focusing on potential dangerosity of foreign frozen foods and reported detection of viral RNA on various imported food, from diverse countries. Infectivity has not been demonstrated, As stated in a recent chinese paper, « … ignoring potential faecal transmission and the gastrointestinal involvement of SARS-CoV-2 may result in mistakes in attempts to control the pandemic. » Indeed, in recent control measures trying to limit contaminations in Beijing, ar least 3 fecal analysis became mandatory beginning of January.

|
Scooped by
Gilbert C FAURE
January 9, 2021 4:20 AM
|
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in late 2019 in China and is the causative agent of the coronavirus disease 2019 (COVID-19) pandemic. To mitigate the effects of the virus on public health, the economy and society, a vaccine is urgently needed. Here I review the development of vaccines against SARS-CoV-2. Development was initiated when the genetic sequence of the virus became available in early January 2020, and has moved at an unprecedented speed: a phase I trial started in March 2020 and there are currently more than 180 vaccines at various stages of development. Data from phase I and phase II trials are already available for several vaccine candidates, and many have moved into phase III trials. The data available so far suggest that effective and safe vaccines might become available within months, rather than years. The development of vaccines against SARS-CoV-2 is reviewed, including an overview of the development process, the different types of vaccine candidate, and data from animal studies as well as phase I and II clinical trials in humans.

|
Scooped by
Gilbert C FAURE
December 22, 2020 3:15 AM
|

|
Scooped by
Gilbert C FAURE
December 9, 2020 9:11 AM
|
According to the scientists, including those from Sorbonne University in Paris, the IgA antibodies found in the mucosa dominate the early response to the SARS-CoV-2 virus compared to other antibodies such as the IgM and IgG.

|
Scooped by
Gilbert C FAURE
December 8, 2020 12:13 PM
|
Studies suggest that SARS-CoV-2-specific IgA antibodies are more neutralising and therefore COVID vaccines should encourage an IgA response.

|
Scooped by
Gilbert C FAURE
November 30, 2020 2:53 PM
|

|
Suggested by
LIGHTING
November 25, 2020 3:42 AM
|
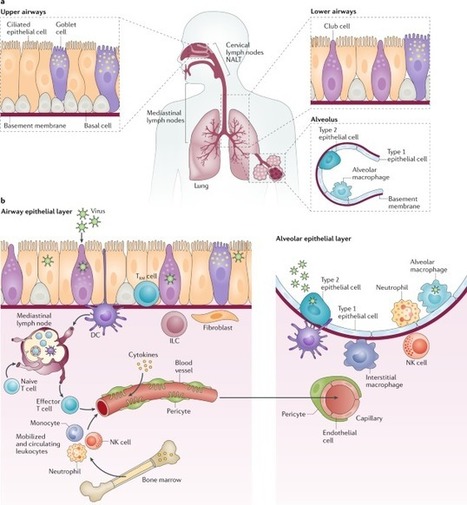
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of coronavirus disease 2019 (COVID-19). Understanding of the fundamental processes underlying the versatile clinical manifestations of COVID-19 is incomplete without comprehension of how different immune cells are recruited to various compartments of virus-infected lungs, and how this recruitment differs among individuals with different levels of disease severity. As in other respiratory infections, leukocyte recruitment to the respiratory system in people with COVID-19 is orchestrated by specific leukocyte trafficking molecules, and when uncontrolled and excessive it results in various pathological complications, both in the lungs and in other organs. In the absence of experimental data from physiologically relevant animal models, our knowledge of the trafficking signals displayed by distinct vascular beds and epithelial cell layers in response to infection by SARS-CoV-2 is still incomplete. However, SARS-CoV-2 and influenza virus elicit partially conserved inflammatory responses in the different respiratory epithelial cells encountered early in infection and may trigger partially overlapping combinations of trafficking signals in nearby blood vessels. Here, we review the molecular signals orchestrating leukocyte trafficking to airway and lung compartments during primary pneumotropic influenza virus infections and discuss potential similarities to distinct courses of primary SARS-CoV-2 infections. We also discuss how an imbalance in vascular activation by leukocytes outside the airways and lungs may contribute to extrapulmonary inflammatory complications in subsets of patients with COVID-19. These multiple molecular pathways are potential targets for therapeutic interventions in patients with severe COVID-19. In this Perspective, Alon and colleagues discuss how insights into immune cell trafficking during pneumotropic influenza virus infections may inform our understanding of immune cell recruitment to the respiratory tract in patients with coronavirus disease 2019 (COVID-19). Moreover, they examine the emerging knowledge of vascular pathologies beyond the lung caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
|

|
Scooped by
Gilbert C FAURE
March 1, 2021 2:06 PM
|

|
Scooped by
Gilbert C FAURE
February 20, 2021 3:58 AM
|
medRxiv - The Preprint Server for Health Sciences

|
Scooped by
Gilbert C FAURE
January 27, 2021 1:38 PM
|
La Chine recourt de façon croissante aux dépistages rectaux pour tester les sujets à risque et les voyageurs arrivant de l'étranger. La méthode rebute.

|
Rescooped by
Gilbert C FAURE
from Virus World
January 17, 2021 2:14 AM
|
Compared with nasal swabs, saliva tests may better reflect infection deep in the lungs. To the known risk factors for developing severe COVID-19—age, male sex, or any of a series of underlying conditions—a new study adds one more: high levels of the virus in your saliva. Standard COVID-19 tests sample the nasal passage. But several new tests look for SARS-CoV-2, the pandemic coronavirus, in saliva, and the new work finds a striking correlation between high virus levels there and later hospitalization or death. If the results are confirmed, saliva tests could help doctors prioritize which patients in the early stages of the disease should receive medicines that drive down levels of the virus. “I thought it was pretty striking,” says Shane Crotty, a virologist at the La Jolla Institute for Immunology, who was not involved with the research. Crotty notes the results suggest virus levels in saliva reflect viral load deep in the lungs, where the disease does much of its damage in severe cases. “That is a fundamentally valuable insight,” Crotty says. The new work isn’t the first to link the body’s coronavirus load and disease outcome. Several research groups have found a correlation between high viral levels in the nasal passages at the time of a patient’s hospital admission and ultimate disease severity. But other groups have failed to find that same link. The standard test to detect SARS-CoV-2 samples nasal mucus using nasopharyngeal (NP) swabs. The procedure is unpleasant, but it is the customary way to sample respiratory pathogens. In recent months, however, several research groups have developed and received emergency use authorization from the U.S. Food and Drug Administration for tests detecting SARS-CoV-2 in saliva. Yale University researchers were among the first, and the university’s hospitals have been using both saliva and NP swab tests. In both cases, labs analyze the samples using quantitative reverse transcription polymerase chain reaction tests, which can detect genetic material from SARS-CoV-2 and quantify the number of viral particles in each milliliter of sample. Researchers led by Akiko Iwasaki, an immunologist at Yale, compared viral loads in saliva and NP swabs from 154 patients and 109 people without the virus. They divided the patients into groups that had low, medium, and high viral loads as determined by both types of test. Then they compared those results with the severity of symptoms the patients developed later. They found that patients who developed severe disease, were hospitalized, or died were more likely to have had high virus loads in their saliva tests, but not in their NP swabs. Viral load in both saliva and nasal mucus declined over time in patients who recovered, but not in those who died. When Iwasaki and her colleagues reviewed patients’ electronic medical records for markers of disease in the blood, they found that high saliva viral loads correlated with high levels of immune signals such as cytokines and chemokines, nonspecific molecules that ramp up in response to viral infections and have been linked to tissue damage. People with more virus in their saliva also gradually lost certain cells that mount an immune response against viral targets, had lower levels of antibodies targeting the spike protein that the virus uses to enter cells, and were slower to develop the strong immune response needed to knock down the virus in cases where they recovered. The team’s results appeared on 10 January in a preprint that has not been peer reviewed. Iwasaki and her colleagues argue that saliva may be a better predictor of disease outcome than nasal mucus because the latter comes from the upper respiratory tract, whereas severe disease is associated with damage deep in the lungs. “Saliva may better represent what is going on in the lower respiratory tract,” Iwasaki says, because cilia lining the respiratory tract naturally move mucus up from the lungs into the throat, where it mixes with saliva; coughs have the same effect. The results don’t have enough statistical power to reveal how much more likely a person with a high saliva viral load is to develop severe COVID-19, Iwasaki says. She is also eager for other groups to replicate the results, especially because efforts to link high NP swab viral loads with disease progression have had mixed results. If other research confirms the finding, “it would clear away a lot of the fog” around this disease, Crotty says. Monica Gandhi, an infectious disease expert at the University of California, San Francisco, adds that if saliva tests are predictive, they could help doctors identify patients to treat early with either antibodies to reduce viral load or steroids to tamp down overactive nonspecific immune responses. Saliva tests are cheaper and easier than NP tests, but much less widely available. So confirmation of the new results could bolster efforts to make saliva tests more readily available, says Sri Kosuri, CEO of Octant, Inc., a biotech company. “If this study happened in March, we’d be talking about whether we should be doing NP testing at all,” Kosuri says. Preprint available in medRxiv (Jan. 10, 2021): https://doi.org/10.1101/2021.01.04.21249236
Via Juan Lama

|
Scooped by
Gilbert C FAURE
January 13, 2021 10:25 AM
|
Answering this question will take us one step closer to our new normal.

|
Scooped by
Gilbert C FAURE
January 11, 2021 1:04 PM
|
medRxiv - The Preprint Server for Health Sciences

|
Scooped by
Gilbert C FAURE
January 10, 2021 4:36 AM
|
It has been reported that SARS-CoV-2 may use ACE2 as a receptor to gain entry into human cells, in a way similar to that of SARS-CoV. Analyzing the distribution and expression level of ACE2 may therefore help reveal underlying mechanisms of viral susceptibility ...

|
Scooped by
Gilbert C FAURE
January 8, 2021 2:50 PM
|

|
Scooped by
Gilbert C FAURE
December 9, 2020 9:12 AM
|
Spread of COVID-19 in all of the world caused the warning alert from WHO. It began from China and was the reason of many death through the world since 2019 December. Elders and people with previous diseases such as IgA deficiency [IgAD] are more susceptible to get COVID family.

|
Scooped by
Gilbert C FAURE
December 8, 2020 12:28 PM
|

|
Scooped by
Gilbert C FAURE
December 1, 2020 4:31 AM
|
The mucosal immune system is the largest component of the entire immune system, having evolved to provide protection at the main sites of infectious threat: the mucosae. As SARS-CoV-2 initially infects the upper respiratory tract, its first interactions with the immune system must occur predominantly at the respiratory mucosal surfaces, during both inductive and effector phases of the response. However, almost all studies of the immune response in COVID-19 have focused exclusively on serum antibodies and systemic cell-mediated immunity including innate responses. This article proposes that there is a significant role for mucosal immunity and for secretory as well as circulating IgA antibodies in COVID-19, and that it is important to elucidate this in order to comprehend especially the asymptomatic and mild states of the infection, which appear to account for the majority of cases. Moreover, it is possible that mucosal immunity can be exploited for beneficial diagnostic, therapeutic, or prophylactic purposes.

|
Scooped by
Gilbert C FAURE
November 26, 2020 8:21 AM
|
The distal lung contains terminal bronchioles and alveoli that facilitate gas exchange. Three-dimensional in vitro human distal lung culture systems would strongly facilitate investigation of pathologies including interstitial lung disease, cancer, and SARS-CoV-2-associated COVID-19 pneumonia. We generated long-term feeder-free, chemically defined culture of distal lung progenitors as organoids derived from single adult human alveolar epithelial type II (AT2) or KRT5+ basal cells. AT2 organoids exhibited AT1 transdifferentiation potential while basal cell organoids developed lumens lined by differentiated club and ciliated cells. Single cell analysis of basal organoid KRT5+ cells revealed a distinct ITGA6+ITGB4+ mitotic population whose proliferation further segregated to a TNFRSF12Ahi subfraction comprising ~10% of KRT5+ basal cells, residing in clusters within terminal bronchioles and exhibiting enriched clonogenic organoid growth activity. Distal lung organoids were created with apical-out polarity to display ACE2 on the exposed external surface, facilitating SARS-CoV-2 infection of AT2 and basal cultures and identifying club cells as a novel target population. This long-term, feeder-free organoid culture of human distal lung, coupled with single cell analysis, identifies unsuspected basal cell functional heterogeneity and establishes a facile in vitro organoid model for human distal lung infections including COVID-19-associated pneumonia.
|
 Your new post is loading...
Your new post is loading...
 Your new post is loading...
Your new post is loading...