Your new post is loading...
 Your new post is loading...

|
Scooped by
Gilbert C FAURE
March 1, 2024 5:27 AM
|
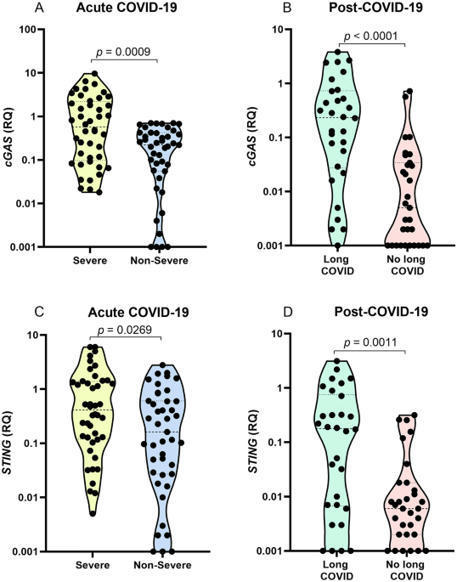
The cGAS-STING pathway appears to contribute to dysregulated inflammation during coronavirus disease 2019 (COVID-19); however, inflammatory factors related to long COVID are still being investigated. In the present study, we evaluated the association of cGAS and STING gene expression levels and plasma IFN-α, TNF-α and IL-6 levels with COVID-19 severity in acute infection and long COVID, based on analysis of blood samples from 148 individuals, 87 with acute COVID-19 and 61 in the post-COVID-19 period. Quantification of gene expression was performed by real-time PCR, and cytokine levels were quantified by ELISA and flow cytometry. In acute COVID-19, cGAS, STING, IFN-α, TNF-α, and IL-6 levels were higher in patients with severe disease than in those with nonsevere manifestations (p < 0.05). Long COVID was associated with elevated cGAS, STING and IFN-α levels (p < 0.05). Activation of the cGAS-STING pathway may contribute to an intense systemic inflammatory state in severe COVID-19 and, after infection resolution, induce an autoinflammatory disease in some tissues, resulting in long COVID.

|
Scooped by
Gilbert C FAURE
August 20, 2023 5:18 AM
|

|
Rescooped by
Gilbert C FAURE
from Virus World
July 1, 2023 1:04 PM
|
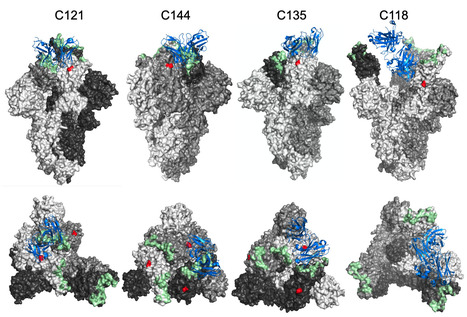
The glycosylation of viral envelope proteins can play important roles in virus biology and immune evasion. The spike (S) glycoprotein of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) includes 22 N-linked glycosylation sequons and 17 O-linked glycosites. Here, we investigated the effect of individual glycosylation sites on SARS-CoV-2 S function in pseudotyped virus infection assays and on sensitivity to monoclonal and polyclonal neutralizing antibodies. In most cases, removal of individual glycosylation sites decreased the infectiousness of the pseudotyped virus. For glycosylation mutants in the N-terminal domain (NTD) and the receptor binding domain (RBD), reduction in pseudotype infectivity was predicted by a commensurate reduction in the level of virion-incorporated spike protein. Notably, the presence of a glycan at position N343 within the RBD had diverse effects on neutralization by RBD-specific monoclonal antibodies (mAbs) cloned from convalescent individuals. The N343 glycan reduced overall sensitivity to polyclonal antibodies in plasma from COVID-19 convalescent individuals, suggesting a role for SARS-CoV-2 spike glycosylation in immune evasion. However, vaccination of convalescent individuals produced neutralizing activity that was resilient to the inhibitory effect of the N343 glycan. Preprint at bioRxiv (June 30, 2023): https://doi.org/10.1101/2023.06.30.547241
Via Juan Lama

|
Scooped by
Gilbert C FAURE
December 8, 2022 11:24 AM
|
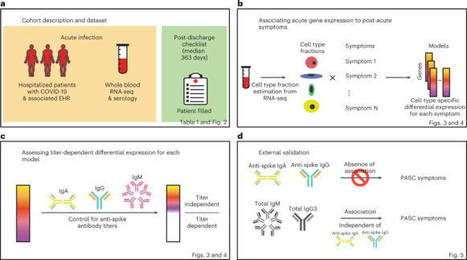
Post-acute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are debilitating, clinically heterogeneous and of unknown molecular etiology. A transcriptome-wide investigation was performed in 165 acutely infected hospitalized individuals who were followed clinically into the post-acute period. Distinct gene expression signatures of post-acute sequelae were already present in whole blood during acute infection, with innate and adaptive immune cells implicated in different symptoms. Two clusters of sequelae exhibited divergent plasma-cell-associated gene expression patterns. In one cluster, sequelae associated with higher expression of immunoglobulin-related genes in an anti-spike antibody titer-dependent manner. In the other, sequelae associated independently of these titers with lower expression of immunoglobulin-related genes, indicating lower non-specific antibody production in individuals with these sequelae. This relationship between lower total immunoglobulins and sequelae was validated in an external cohort. Altogether, multiple etiologies of post-acute sequelae were already detectable during SARS-CoV-2 infection, directly linking these sequelae with the acute host response to the virus and providing early insights into their development. Transcriptomic analyses of acute phase whole blood from a large cohort of patients with COVID-19 identify molecular determinants of post-infection long-term sequelae.

|
Scooped by
Gilbert C FAURE
March 27, 2022 4:55 AM
|
The coronavirus disease 2019 (COVID-19) pandemic, causing considerable mortality and morbidity worldwide, has fully engaged the biomedical community in attempts to elucidate the pathophysiology of COVID-19 and develop robust therapeutic strategies.

|
Rescooped by
Gilbert C FAURE
from Virus World
January 31, 2022 3:43 AM
|
The entry of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) into human cells is an essential step for virus transmission and development of COVID 19. Although the lung epithelial cells are its initial target, SARS-CoV-2 also can infect endothelial cells. Endothelial cells are the major constituents of the vascular system and cardiovascular complication is a hallmark of severe COVID-19. Angiotensin-converting enzyme 2 (ACE2) is the entry receptor for SARS-CoV-2. However, the possible involvement of other cellular components in the viral entry is not fully understood. A team of researchers from the Boston University School of Medicine (BUSM) has identified extracellular vimentin as an attachment factor that facilitates SARS-CoV-2 entry into human cells. Vimentin is a structural protein that is widely expressed in the cells of mesenchymal origin such as endothelial cells and a potential novel target against SARS-CoV-2, which could block the infection of the SARS-CoV-2. "Severe endothelial injury, vascular thrombosis, and obstruction of alveolar capillaries (tiny air sacs scattered throughout the lungs) are common features of severe COVID-19. Identification of vimentin as a host attachment factor for SARS-CoV-2 can provide new insight into the mechanism of SARS-CoV-2 infection of the vascular system and can lead to the development of novel treatment strategies," said corresponding author Nader Rahimi, Ph.D., associate professor of pathology & laboratory medicine at BUSM. The researchers used liquid chromatography–tandem mass spectrometry (LC-MS/MS) and identified vimentin as a protein that binds to the SARS-CoV-2 spike (S) protein and facilitates SARS-CoV-2 infection. They also found that depletion of vimentin significantly reduces SARS-CoV-2 infection of human endothelial cells. In contrast, over-expression of vimentin with ACE2 significantly increased the infection rate. "More importantly, we saw that the CR3022 antibody inhibited the binding of vimentin with CoV-2-S-protein, and neutralized SARS-CoV-2 entry into human cells," explained Rahimi. Findings Published in P.N.A.S. (Jan. 25, 2022): https://doi.org/10.1073/pnas.2113874119
Via Juan Lama

|
Scooped by
Gilbert C FAURE
January 16, 2022 11:22 AM
|
Cross-reactive immune responses to SARS-CoV-2 have been observed in pre-pandemic cohorts and proposed to contribute to host protection. Here we assess 52 COVID-19 household contacts to capture immune responses at the earliest timepoints after SARS-CoV-2 exposure. Using a dual cytokine FLISpot assay on peripheral blood mononuclear cells, we enumerate the frequency of T cells specific for spike, nucleocapsid, membrane, envelope and ORF1 SARS-CoV-2 epitopes that cross-react with human endemic coronaviruses. We observe higher frequencies of cross-reactive (p = 0.0139), and nucleocapsid-specific (p = 0.0355) IL-2-secreting memory T cells in contacts who remained PCR-negative despite exposure (n = 26), when compared with those who convert to PCR-positive (n = 26); no significant difference in the frequency of responses to spike is observed, hinting at a limited protective function of spike-cross-reactive T cells. Our results are thus consistent with pre-existing non-spike cross-reactive memory T cells protecting SARS-CoV-2-naïve contacts from infection, thereby supporting the inclusion of non-spike antigens in second-generation vaccines. While cross-reactive immunity between human coronavirus and SARS-CoV-2 may contribute to host protection, validating evidences are still scarce. Here the authors assess a cohort of 52 donors with immediate-early contact with SARS-CoV-2 to correlate higher frequency of cross-reactive T cells with lower infection rate.

|
Scooped by
Gilbert C FAURE
December 8, 2021 6:42 AM
|
Immunological memory is a hallmark of adaptive immunity and facilitates an accelerated and enhanced immune response upon re-infection with the same pathogen1,2. Since the outbreak of the ongoing coronavirus disease 19 (COVID-19) pandemic, a key question has focused on which severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-specific T cells stimulated during acute infection give rise to long-lived memory T cells3. Using spectral flow cytometry combined with cellular indexing of transcriptomes and T cell receptor (TCR) sequencing we longitudinally characterize individual SARS-CoV-2-specific CD8+ T cells of COVID-19 patients from acute infection to one year into recovery and find a distinct signature identifying long-lived memory CD8+ T cells. SARS-CoV-2-specific memory CD8+ T cells persisting one year after acute infection express CD45RA, interleukin-7 receptor α (CD127), and T cell factor-1 (TCF1), but they maintain low CCR7, thus resembling CD45RA+ effector-memory T (TEMRA) cells. Tracking individual clones of SARS-CoV-2-specific CD8+ T cells, we reveal that an interferon signature marks clones giving rise to long-lived cells, whereas prolonged proliferation and mammalian target of rapamycin (mTOR) signaling are associated with clonal disappearance from the blood. Collectively, we describe a transcriptional signature that marks long-lived, circulating human memory CD8+ T cells following an acute virus infection.

|
Scooped by
Gilbert C FAURE
October 13, 2021 10:57 AM
|
Virus entry, consisting of attachment to and penetration into the host target cell, is the first step of the virus life cycle and is a critical ‘do or die’ event that governs virus emergence in host populations. Most antiviral vaccines induce neutralizing antibodies that prevent virus entry into cells. However, while the prevention of virus invasion by humoral immunity is well appreciated, considerably less is known about the immune defences present within cells (known as intrinsic immunity) that interfere with virus entry. The interferon-induced transmembrane (IFITM) proteins, known for inhibiting fusion between viral and cellular membranes, were once the only factors known to restrict virus entry. However, the progressive development of genetic and pharmacological screening platforms and the onset of the COVID-19 pandemic have galvanized interest in how viruses infiltrate cells and how cells defend against it. Several host factors with antiviral potential are now implicated in the regulation of virus entry, including cholesterol 25-hydroxylase (CH25H), lymphocyte antigen 6E (LY6E), nuclear receptor co-activator protein 7 (NCOA7), interferon-γ-inducible lysosomal thiol reductase (GILT), CD74 and ARFGAP with dual pleckstrin homology domain-containing protein 2 (ADAP2). This Review summarizes what is known and what remains to be understood about the intrinsic factors that form the first line of defence against virus infection. Besides neutralizing antibodies, viruses face a range of cell-intrinsic inhibitors that are specialized to limit virus entry into host cells. Majdoul and Compton describe the mechanisms of action of the cellular factors providing this important first line of defence against virus infection, including infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).

|
Scooped by
Gilbert C FAURE
August 27, 2021 6:36 AM
|
Among the many activities attributed to the type I interferon (IFN) multigene family, their roles as mediators of the antiviral immune response have emerged as important components of the host response to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection.

|
Rescooped by
Gilbert C FAURE
from Virus World
June 24, 2021 3:01 AM
|
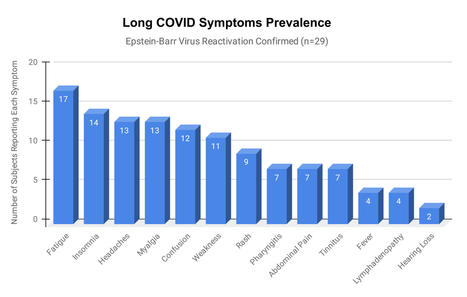
Epstein-Barr virus (EBV) reactivation resulting from the inflammatory response to coronavirus infection may be the cause of previously unexplained long COVID symptoms—such as fatigue, brain fog, and rashes—that occur in approximately 30% of patients after recovery from initial COVID-19 infection. The first evidence linking EBV reactivation to long COVID, as well as an analysis of long COVID prevalence, is outlined in a new long COVID study published in the journal Pathogens. "We ran EBV antibody tests on recovered COVID-19 patients, comparing EBV reactivation rates of those with long COVID symptoms to those without long COVID symptoms," said lead study author Jeffrey E. Gold of World Organization. "The majority of those with long COVID symptoms were positive for EBV reactivation, yet only 10% of controls indicated reactivation." The researchers began by surveying 185 randomly selected patients recovered from COVID-19 and found that 30.3% had long term symptoms consistent with long COVID after initial recovery from SARS-CoV-2 infection. This included several patients with initially asymptomatic COVID-19 cases who later went on to develop long COVID symptoms. The researchers then found, in a subset of 68 COVID-19 patients randomly selected from those surveyed, that 66.7% of long COVID subjects versus 10% of controls were positive for EBV reactivation based on positive EBV early antigen-diffuse (EA-D) IgG or EBV viral capsid antigen (VCA) IgM titers. The difference was significant (p < 0.001, Fisher's exact test). "We found similar rates of EBV reactivation in those who had long COVID symptoms for months, as in those with long COVID symptoms that began just weeks after testing positive for COVID-19," said coauthor David J. Hurley, Ph.D., a professor and molecular microbiologist at the University of Georgia. "This indicated to us that EBV reactivation likely occurs simultaneously or soon after COVID-19 infection." The relationship between SARS-CoV-2 and EBV reactivation described in this study opens up new possibilities for long COVID diagnosis and treatment. The researchers indicated that it may be prudent to test patients newly positive for COVID-19 for evidence of EBV reactivation indicated by positive EBV EA-D IgG, EBV VCA IgM, or serum EBV DNA tests. If patients show signs of EBV reactivation, they can be treated early to reduce the intensity and duration of EBV replication, which may help inhibit the development of long COVID. "As evidence mounts supporting a role for EBV reactivation in the clinical manifestation of acute COVID-19, this study further implicates EBV in the development of long COVID," said Lawrence S. Young, Ph.D., a virologist at the University of Warwick, and Editor-in-Chief of Pathogens. "If a direct role for EBV reactivation in long COVID is supported by further studies, this would provide opportunities to improve the rational diagnosis of this condition and to consider the therapeutic value of anti-herpesvirus agents such as ganciclovir." Original findings published in Pathogens (June 10, 2021): https://doi.org/10.3390/pathogens10060763
Via Juan Lama

|
Scooped by
Gilbert C FAURE
May 24, 2021 1:08 PM
|
Long-lived bone marrow plasma cells (BMPCs) are a persistent and essential source of protective antibodies1–7. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) convalescent individuals have a significantly lower risk of reinfection8–10. Nonetheless, it has been reported that anti-SARS-CoV-2 serum antibodies experience rapid decay in the first few months after infection, raising concerns that long-lived BMPCs may not be generated and humoral immunity against this virus may be short-lived11–13. Here we demonstrate that in patients who experienced mild infections (n=77), serum anti-SARS-CoV-2 spike (S) antibodies decline rapidly in the first 4 months after infection and then more gradually over the following 7 months, remaining detectable at least 11 months after infection. Anti-S antibody titers correlated with the frequency of S-specific BMPCs obtained from bone marrow aspirates of 18 SARS-CoV-2 convalescent patients 7 to 8 months after infection. S-specific BMPCs were not detected in aspirates from 11 healthy subjects with no history of SARS-CoV-2 infection. We demonstrate that S-binding BMPCs are quiescent, indicating that they are part of a long-lived compartment. Consistently, circulating resting memory B cells directed against the S protein were detected in the convalescent individuals. Overall, we show that SARS-CoV-2 infection induces a robust antigen-specific, long-lived humoral immune response in humans.

|
Scooped by
Gilbert C FAURE
April 15, 2021 10:36 AM
|
Emerging studies indicate that some coronavirus disease 2019 (COVID-19) patients suffer from persistent symptoms, including breathlessness and chronic…
|

|
Scooped by
Gilbert C FAURE
January 15, 2024 5:07 AM
|
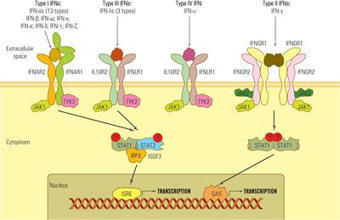
Mammalians sense antigenic messages from infectious agents that penetrate the respiratory and digestive epithelium, as well as signals from damaged host cells through membrane and cytosolic receptors. The transduction of these signals triggers a personalized response, depending on the nature of the stimulus and the host’s genetics, physiological condition, and comorbidities. Interferons (IFNs) are the primary effectors of the innate immune response, and their synthesis is activated in most cells within a few hours after pathogen invasion. IFNs are primarily synthesized in infected cells, but their anti-infective effect is extended to the neighboring cells by autocrine and paracrine action. The emergence of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pandemic in 2019 was a stark reminder of the potential threat posed by newly emerging viruses. This pandemic has also triggered an overwhelming influx of research studies aiming to unveil the mechanisms of protective versus pathogenic host immune responses induced by SARS‐CoV‐2. The purpose of this review is to describe the role of IFNs as vital players in the battle against SARS‐CoV-2 infection. We will briefly characterize and classify IFNs, present the inductors of IFN synthesis, their sensors, and signaling pathways, and then discuss the role of IFNs in controlling the evolution of SARS-CoV-2 infection and its clinical outcome. Finally, we will present the perspectives and controversies regarding th

|
Scooped by
Gilbert C FAURE
August 15, 2023 5:03 AM
|
Long COVID is an often debilitating illness that occurs in at least 10% of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections. More than 200 symptoms have been identified with impacts on multiple organ systems. At least 65 million individuals worldwide are estimated to have long COVID, with cases increasing daily. Biomedical research has made substantial progress in identifying various pathophysiological changes and risk factors and in characterizing the illness; further, similarities with other viral-onset illnesses such as myalgic encephalomyelitis/chronic fatigue syndrome and postural orthostatic tachycardia syndrome have laid the groundwork for research in the field. In this Review, we explore the current literature and highlight key findings, the overlap with other conditions, the variable onset of symptoms, long COVID in children and the impact of vaccinations. Although these key findings are critical to understanding long COVID, current diagnostic and treatment options are insufficient, and clinical trials must be prioritized that address leading hypotheses. Additionally, to strengthen long COVID research, future studies must account for biases and SARS-CoV-2 testing issues, build on viral-onset research, be inclusive of marginalized populations and meaningfully engage patients throughout the research process. Long COVID is an often debilitating illness of severe symptoms that can develop during or following COVID-19. In this Review, Davis, McCorkell, Vogel and Topol explore our knowledge of long COVID and highlight key findings, including potential mechanisms, the overlap with other conditions and potential treatments. They also discuss challenges and recommendations for long COVID research and care.

|
Scooped by
Gilbert C FAURE
January 11, 2023 12:48 PM
|
bioRxiv - the preprint server for biology, operated by Cold Spring Harbor Laboratory, a research and educational institution

|
Scooped by
Gilbert C FAURE
May 21, 2022 9:52 AM
|
interferon, friend or foe

|
Scooped by
Marcelo de Carvalho Bittencourt
February 8, 2022 12:23 PM
|
Broadly neutralizing antibodies (bnAbs) to coronaviruses (CoVs) are valuable in their own right as prophylactic and therapeutic reagents to treat diverse CoVs and, importantly, as templates for rat...

|
Scooped by
Gilbert C FAURE
January 18, 2022 6:44 AM
|
Hyperactivation of the complement and coagulation systems is recognized as part of the clinical syndrome of COVID-19. Here we review systemic complement activation and local complement activation in response to the causative virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and their currently known relationships to hyperinflammation and thrombosis. We also provide an update on early clinical findings and emerging clinical trial evidence that suggest potential therapeutic benefit of complement inhibition in severe COVID-19. Hyperactivation of the complement system has been implicated in the pathology of COVID-19. Here the authors bring together the latest information on the role of complement in COVID-19 and progress in targeting complement components for treatment of severe disease.

|
Rescooped by
Gilbert C FAURE
from Veille Coronavirus - Covid-19
January 14, 2022 2:58 AM
|
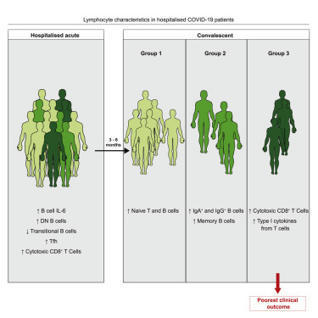
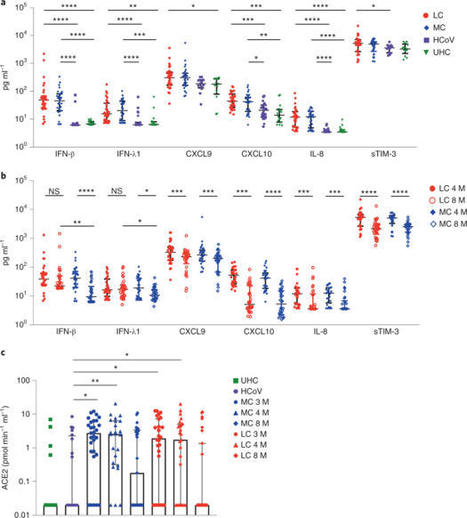
A proportion of patients surviving acute coronavirus disease 2019 (COVID-19) infection develop post-acute COVID syndrome (long COVID (LC)) lasting longer than 12 weeks. Here, we studied individuals with LC compared to age- and gender-matched recovered individuals without LC, unexposed donors and individuals infected with other coronaviruses. Patients with LC had highly activated innate immune cells, lacked naive T and B cells and showed elevated expression of type I IFN (IFN-β) and type III IFN (IFN-λ1) that remained persistently high at 8 months after infection. Using a log-linear classification model, we defined an optimal set of analytes that had the strongest association with LC among the 28 analytes measured. Combinations of the inflammatory mediators IFN-β, PTX3, IFN-γ, IFN-λ2/3 and IL-6 associated with LC with 78.5–81.6% accuracy. This work defines immunological parameters associated with LC and suggests future opportunities for prevention and treatment. Phetsouphanh and colleagues show that individuals with long COVID have persistent activation of the innate and adaptive immune system at 8 months after infection and define a set of analytes associated with long COVID.
Via HAS-veille

|
Scooped by
Gilbert C FAURE
October 28, 2021 3:07 AM
|
5 juillet 2021 Une équipe de scientifiques internationaux a récemment identifié des anticorps ultrapuissants anti-coronavirus 2 (SRAS-CoV-2) anti-syndrome respiratoire aigu sévère provenant de donneurs convalescents. Les anticorps sont capables de neutraliser une large gamme de variantes du SRAS-CoV-2, même à des concentrations sous-nanomolaires. De plus, les combinaisons de ces anticorps réduisent le risque de générer des mutants d'échappement in vitro. L'étude a été publiée le 13 août 2021 dans la revue Science. https://www.science.org/doi/full/10.112 ... ce.abh1766 Sur le fond Le coronavirus 2 du syndrome respiratoire aigu sévère (SRAS-CoV-2), l'agent pathogène responsable de la maladie à coronavirus 2019 (COVID-19), est un virus à ARN simple brin enveloppé de sens positif appartenant à la famille des bêta-coronavirus humains. La glycoprotéine de pointe sur l'enveloppe virale est composée de deux sous-unités S1 et S2. Dont, la sous-unité S1 se lie directement au récepteur de l'enzyme de conversion de l'angiotensine 2 (ACE2) de la cellule hôte via le domaine de liaison au récepteur (RBD) pour initier le processus d'entrée virale. La majorité des anticorps thérapeutiques contre le SRAS-CoV-2 ont été conçus sur la base de la séquence de protéine de pointe native trouvée dans la souche Wuhan originale du SRAS-CoV-2. Ainsi, de nouvelles variantes virales avec de multiples mutations de la protéine de pointe peuvent probablement développer une résistance contre ces anticorps. Dans ce contexte, des études ont montré que les anticorps développés en réponse aux vaccins COVID-19 actuellement disponibles ont moins d'efficacité pour neutraliser les nouvelles variantes préoccupantes (COV) du SRAS-CoV, notamment B.1.1.7, B.1.351, P1 et B.1.617.2. Dans la présente étude, les scientifiques ont isolé et caractérisé des anticorps anti-pic RBD de patients guéris du COVID-19. Identification des anticorps Les anticorps ont été isolés de quatre donneurs convalescents infectés par la souche Washington-1 (WA-1) du SRAS-CoV-2. La séquence de pointe dans la souche WA-1 est similaire à la séquence de pointe dans la souche originale de Wuhan. Les cellules B isolées à partir d'échantillons de sang provenant de donneurs ont été triées pour l'identification des anticorps. Cela a conduit à l'identification de quatre anticorps neutralisants puissants ciblant le pic RBD. Ces anticorps ont montré une forte affinité pour le pic SRAS-CoV-2 même à des concentrations nanomolaires. Pour déterminer si les anticorps hautement puissants pouvaient bloquer l'ACE2 - la liaison aux pointes, des tests d'interférométrie par compétition ACE2 et de liaison à la surface cellulaire ont été effectués. Les résultats ont révélé que sur 4 anticorps, deux liés aux RBD en « position haute » et deux liés aux RBD en « position basse ». De plus, trois anticorps sur quatre bloquaient directement l'interaction RBD - ACE2, et un inhibait indirectement l'interaction par encombrement stérique - le ralentissement des réactions chimiques dû à l'encombrement stérique. Neutralisation médiée par les anticorps Tous les anticorps expérimentaux ont montré une puissance significativement plus élevée dans la neutralisation des variants contenant la mutation D614G que la souche WA-1. Une analyse plus poussée avec des particules lentivirales pseudotypées avec des variantes à pointes a indiqué que les anticorps maintiennent une puissance élevée pour neutraliser un ensemble diversifié de 10 variantes à pointes. Il est important de noter que trois des quatre anticorps expérimentaux ont montré une grande efficacité pour neutraliser 13 variantes circulantes préoccupantes/intéressantes du SRAS-CoV-2, notamment B.1.1.7, B.1.351, B.1.427, B.1.429, B.1.526, P.1, P.2, B.1.617.1 et B.1.617.2. Analyse structurale et fonctionnelle des anticorps Des analyses au microscope cryoélectronique des structures du complexe anticorps-antigène ont révélé que deux anticorps avec le pouvoir de neutralisation le plus élevé se lient à la protéine de pointe avec tous les RBD en « position haute ». D'autres analyses structurelles ont révélé que les modes de liaison à l'épitope des anticorps sont responsables d'un pouvoir neutralisant élevé contre les COV du SRAS-CoV-2. La capacité de liaison et de neutralisation des anticorps a été affectée négativement par trois mutations de pointe, dont F486R, N487R et Y489R. Résistance aux anticorps Une pression de sélection d'anticorps a été appliquée à la souche WA-1 pour identifier les mutations potentielles d'échappement qui peuvent apparaître au cours de l'infection virale. La pression de sélection positive a été appliquée en incubant le virus avec des concentrations croissantes des anticorps pour déclencher la résistance aux anticorps. Dans deux des anticorps les plus puissants, l'un était affecté négativement par une seule mutation F486S et l'autre était affecté par les mutations F486L, N487D et Q493R. Cependant, la mutation Q493R a montré un impact négligeable sur la liaison et la neutralisation. Une analyse plus poussée a révélé que ces mutations d'échappement sont principalement absentes dans les variantes virales circulantes, indiquant l'absence de pression de sélection. En effectuant plusieurs cycles de sélection à l'aide de traitements combinés avec deux anticorps, il a été observé que les combinaisons d'anticorps pourraient réduire le risque d'acquisition de mutations d'échappement et le développement ultérieur de variantes virales résistantes. Source: News Medical et merci à QcLiLi, dans Twitter En référence à "Wang L. 2021. Anticorps ultrapuissants contre des variantes diverses et hautement transmissibles du SRAS-CoV-2. Sciences". -- -- --

|
Scooped by
Gilbert C FAURE
September 14, 2021 5:17 AM
|
The introduction of vaccines has inspired new hope in the battle against SARS-CoV-2. However, the emergence of viral variants, in the absence of potent antivirals, has left the world struggling wit...

|
Scooped by
Gilbert C FAURE
August 2, 2021 5:49 AM
|
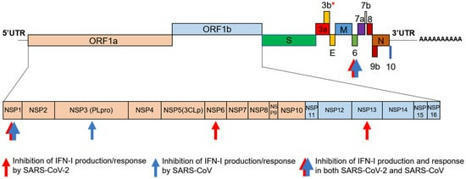
Scientists are unpicking the life cycle of SARS-CoV-2 and how the virus uses tricks to evade detection.

|
Scooped by
Gilbert C FAURE
June 8, 2021 3:08 PM
|
In this study, we found that patients suffering from severe COVID-19 presented with immature, activated blood neutrophils and were characterized by elevated plasma levels of G-CSF and CXCL8 tha

|
Scooped by
Gilbert C FAURE
May 2, 2021 4:42 AM
|
Title: COVID-19 and your immune system Date/Time: Tuesday 4 May 2021 – 18:00 to 19:00 Location: GoToWebinar Register now Description: The UK Coronavirus Immuno...
|


 Your new post is loading...
Your new post is loading...