Your new post is loading...
 Your new post is loading...

|
Scooped by
I2BC Paris-Saclay
April 29, 2:50 AM
|
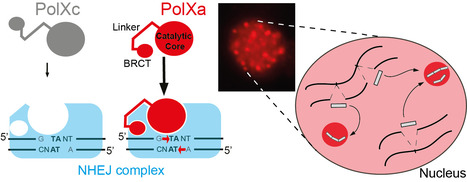
Paramecium PolX DNA polymerases keep the genome safe during programmed rearrangements
The expansion of PolX DNA polymerases in the ciliate Paramecium tetraurelia has favored the functional diversification of a subset of these enzymes, with enhanced nuclear localization in DNA repair foci during programmed DNA elimination. During its sexual cycle, Paramecium massively rearranges its genome by removing tens of thousands of internal eliminated sequences (IESs) from its developing somatic nucleus. This process involves the introduction of programmed DNA double-strand breaks (DSBs), followed by efficient DSB repair using the non-homologous end joining (NHEJ) pathway. In this system, DSB repair requires not only the core NHEJ factors Ku70/80 and Xrcc4/Lig4, but also additional enzymes that accurately process the 4-base 5′-overhangs of the broken DNA ends created at IES excision sites. In this study, we identify four orthologs of human DNA polymerase lambda in P. tetraurelia —PolXa, PolXb, PolXc, and PolXd— as key players in repairing IES excision junctions. Cells lacking these PolX enzymes accumulate genome-wide DNA damage, including unrepaired double-strand breaks, small deletions, and retained IESs. All four PolX proteins can process DNA ends, but PolXa and PolXb are specifically produced during programmed genome rearrangement and possess a unique internal linker region that enhances their nuclear localization. In contrast to PolXc, we show that PolXa concentrates in nuclear foci along with core NHEJ factors and a Dicer-like enzyme involved in producing IES-specific small RNAs that regulate IES excision. We propose that these foci are the sites where excised IESs are ligated into concatemers, which serve as templates for small RNA production, linking DNA repair to RNA-mediated regulation of genome dynamics. More information: https://academic.oup.com/nar/article/53/7/gkaf286/8113560 Contact: Mireille Bétermier mireille.betermier@i2bc-paris-saclay.fr, Julien Bischerour julien.bischerour@i2bc.paris-saclay.fr

|
Scooped by
I2BC Paris-Saclay
April 15, 8:38 AM
|
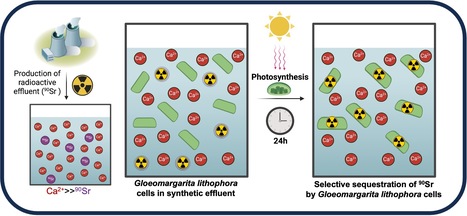
The cyanobacterium G. lithophora is a promising organism for effective bioremediation of waters contaminated with 90Sr
The unicellular cyanobacterium Gloeomargarita lithophora is able to stably sequester and withstand 90Sr and efficiently remove this hazardous anthropogenic radionuclide from aqueous solutions, including a synthetic nuclear effluent. Strontium (Sr) is an alkaline earth metal commonly occurring in nature. In aqueous environments, it prevails as Sr2+ ions form chemically similar to Ca2+. In most organisms, the non-selective Sr intake primarily comes from water and food. In human, it is mainly localized in bones and dental enamel and is involved in the control of bone formation. Radioactive isotopes such as 90Sr are artificially produced by nuclear fission. 90Sr, a b-emitter with a half-life of 28.8 years, is one of the most hazardous anthropogenic radionuclides. It has been massively released into the environment during nuclear weapons testing and nuclear reactor accidents, such as those at Chernobyl and Fukushima. It is also found in radioactive water effluents produced by nuclear power plants, requiring specific treatment before being discharged. In humans, its accumulation in bone tissues increases the risk of cancers. Current physico-chemical techniques for remediating 90Sr traces from effluents are costly and can exhibit a low selectivity for Sr over Ca. Therefore, there is an incentive to develop an alternative method of remediation. In this study, we demonstrate that the cyanobacterium Gloeomargarita lithophora can remove more than 90% of the 90Sr activity that could be found in a nuclear plant effluent within 24 hours. This process occurs through two steps: a first rapid and passive phase of 90Sr sorbtion to the cell surface, followed by an active phase of 90Sr accumulation within the cells, partially driven by photosynthesis. We also showed that this cyanobacterium is able to stably sequester and withstand 90Sr and to efficiently remove this hazardous radionuclide from aqueous solutions, including a synthetic nuclear effluent. These results highlight Gloeomargarita lithophora as a promising solution for effective bioremediation of water contaminated with 90Sr thereby safeguarding the well-being of our ecosystems.
More information : https://doi.org/10.1016/j.jhazmat.2025.138155 Contact : Corinne CASSIER-CHAUVAT Corinne.CASSIER-CHAUVAT@cea.fr

|
Scooped by
I2BC Paris-Saclay
April 11, 5:59 AM
|
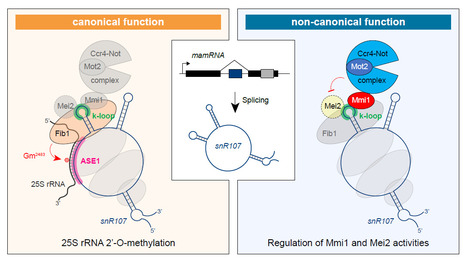
A bifunctional snoRNA guides rRNA 2’-O-methylation and scaffolds gametogenesis effectors
This study uncovers a fission yeast small nucleolar RNA (snoRNA) that guides ribosomal RNA 2’- O-methylation and modulates the activities of RNA-binding proteins involved in gametogenesis, expanding our vision of the non-canonical functions exerted by snoRNAs. Small nucleolar RNAs are non-coding transcripts that guide chemical modifications of RNA substrates and modulate gene expression at the epigenetic and post-transcriptional levels. However, the extent of their regulatory potential and the underlying molecular mechanisms remain poorly understood. In a collaborative work with the I2BC B3S department and NGS facility, the epiRNA-Seq facility in Nancy and the Palancade lab at the Institut Jacques Monod, we have identified a conserved, previously unannotated intronic C/D-box snoRNA, termed snR107, hosted in the fission yeast long non-coding RNA mamRNA and carrying two independent cellular functions. On the one hand, snR107 guides site-specific 25S rRNA 2’-O-methylation and promotes pre-rRNA processing and 60S subunit biogenesis. On the other hand, snR107 associates with the gametogenic RNA-binding proteins Mmi1 and Mei2, mediating their reciprocal inhibition and restricting meiotic gene expression during sexual differentiation. Both functions require distinct cis-motifs within snR107, including a conserved 2’-O-methylation guiding sequence. Together, our results position snR107 as a dual regulator of rRNA modification and gametogenesis effectors, expanding our vision on the non-canonical functions exerted by snoRNAs in cell fate decisions. More Information: https://www.nature.com/articles/s41467-025-58664-y Contact: Mathieu Rougemaille mathieu.rougemaille@i2bc.paris-saclay.fr

|
Scooped by
I2BC Paris-Saclay
April 3, 8:51 AM
|
A monomer–dimer switch modulates the activity of plant adenosine kinase
Novel regulation of adenosine kinase activity in plants Adenosine undergoes ATP-dependent phosphorylation catalyzed by adenosine kinase (ADK). In plants, ADK also phosphorylates cytokinin ribosides, transport forms of the hormone. Here, we investigated the substrate preferences, oligomeric states, and structures of ADKs from moss (Physcomitrella patens) and maize (Zea mays) alongside metabolomic and phenotypic analyses. We showed that dexamethasone-inducible ZmADK overexpressor lines in Arabidopsis can benefit from a higher number of lateral roots and larger root areas under nitrogen starvation. We discovered that maize and moss enzymes can form dimers upon increasing protein concentration, setting them apart from the monomeric human and protozoal ADKs. Structural and kinetic analyses revealed a catalytically inactive unique dimer. Within the dimer, both active sites are mutually blocked. The activity of moss ADKs, exhibiting a higher propensity to dimerize, was 10-fold lower compared with maize ADKs. Two monomeric structures in a ternary complex highlight the characteristic transition from an open to a closed state upon substrate binding. This suggests that the oligomeric state switch can modulate the activity of moss ADKs and probably other plant ADKs. Moreover, dimer association represents a novel negative feedback mechanism, helping to maintain steady levels of adenosine and AMP. More information: https://academic.oup.com/jxb/advance-article/doi/10.1093/jxb/eraf094/8068880 Contact: Solange Morera solange.morera@i2bc.paris-saclay.fr

|
Scooped by
I2BC Paris-Saclay
April 3, 3:43 AM
|
Imagerie-Gif : New TIRF & Phase contrast modality

|
Scooped by
I2BC Paris-Saclay
March 11, 5:05 PM
|
Borders in our chromosomes shape the neighboring domains
Researchers at the I2BC and the Ecole Normale Supérieure found that the borders that create separation between functional domains in our chromosomes have an unexpectedly large influence on those domains themselves. Chromosomes in humans and other mammals are subdivided into “functional neighborhoods” that guide the fidelity of biological processes, including gene regulation and DNA repair. Perturbations of these neighborhoods, resulting in the fusion of adjacent domains, can lead to a variety of diseases, including cancer and developmental disorders.
In a study published in PNAS (Proceedings of the National Academy of Sciences of the USA), scientists from the Noordermeer group at the I2BC, together with their colleagues at the Ecole Normale Supérieure in Paris, report how chromosomal border elements influence the organization of the adjacent functional neighborhoods. By combining genomics-based analysis and biophysical simulations of chromosome structure, they find that the size of these borders is highly diverse, from simple “points” on the chromosome to highly extended zones of transition between neighborhoods. Computer simulations of chromosome behavior of different types of borders in-between revealed an unexpected and far-reaching impact of the borders on their neighboring domains. Rather than creating a static separation, the borders will actively influence the degree of neighborhood mixing due to a previously unrecognized mechanism whereby the borders reel-in the adjacent neighborhoods. At narrow borders, this actively promotes mixing and may cause interference between biological activity on both side of the border. Extended borders buffer against this mechanism by adding additional separation. Border structure can thus constitute a new regulatory layer in the genome to fine-tune biological processes. More information: https://www.pnas.org/doi/10.1073/pnas.2413112122 Contact: Daan Noordermeer daan.noordermeer@i2bc.paris-saclay.fr

|
Scooped by
I2BC Paris-Saclay
March 4, 2:57 AM
|
Ribosome profiling and immunopeptidomics reveal tens of novel conserved HIV-1 ORF encoding T cell antigens.
The translatome of HIV-1 reveals tens of alternative open reading frames (ARF), encoding conserved viral antigens. In vivo, ARF-derived peptides elicit potent HIV-specific poly-functional T cell responses mediated by both CD4+ and CD8+ T cells. T lymphocytes play a pivotal role in controlling human immunodeficiency virus type 1 (HIV-1) infection. Their activation relies on the recognition of viral peptides, or antigens, presented on the surface of infected cells by major histocompatibility complex (MHC) molecules. It is widely assumed that these antigens arise solely from canonical HIV proteins. Previously, we showed that HIV-specific T lymphocytes can also target peptides derived from HIV alternative reading frames (ARFs) presented by MHC molecules. However, evidence for ARFs in the HIV genome had thus far been indirect. In this new study, using ribosome profiling (RiboSeq), we identified the complete HIV-1 translatome in infected CD4⁺ T cells. This approach systematically identified virally encoded mRNA sequences actively translated in HIV-infected cells. We found that the HIV-1 genome contains over one hundred ARFs located either in the 5ʹ untranslated region (5ʹUTR) of classical viral genes or overlapping canonical HIV open reading frames. Using two complementary methods: (1) detecting T lymphocytes specific to ARF-derived peptides in PBMCs from people living with HIV and (2) directly isolating ARF-derived peptides bound to MHC molecules via mass spectrometry–based immunopeptidomics—we demonstrate that HIV ARFs encode novel viral antigens capable of eliciting broad and potent T-cell responses. Our results expand the range of HIV antigens that could be harnessed for vaccine development and may also reveal the existence of microproteins or pseudogenes in the HIV genome.
These findings stem from the work of two complementary teams at I2BC: one led by Olivier Namy, a specialist in protein translation, and the other led by Arnaud Moris, an expert in the immune response to HIV. This collaboration also involved French research and clinical teams and the University of Tübingen in Germany. More information: https://doi.org/10.1038/s41467-025-56773-2 Contact: Arnaud Moris arnaud.moris@i2bc.paris-saclay.fr & Olivier Namy olivier.namy@i2bc.paris-saclay.fr Image: Sonhita Chakraborty

|
Scooped by
I2BC Paris-Saclay
February 12, 9:38 AM
|
The Asgard archaeal origins of Arf family GTPases involved in eukaryotic organelle dynamics
How did the endomembrane system, unique to eukaryotes, emerge from their prokaryotic ancestors? We report that Arf proteins, which are crucial regulators of membrane dynamics in eukaryotes, originated in the archaeal ancestor of eukaryotes, the Asgard Archaea. The evolution of eukaryotes is a fundamental event in the history of life. The closest prokaryotic lineage to eukaryotes, the Asgardarchaeota, encode proteins previously found only in eukaryotes, providing insight into their archaeal ancestor. Eukaryotic cells are characterized by endomembrane organelles, and the Arf family GTPases regulate organelle dynamics by recruiting effector proteins to membranes upon activation. The Arf familyis ubiquitous among eukaryotes, but its origins remain elusive. Here we report a group of prokaryotic GTPases, the ArfRs, which are widely present in Asgardarchaeota. Phylogenetic analyses reveal that eukaryotic Arf family proteins arose from the ArfR group. Expression of representative Asgardarchaeota ArfR proteins in yeast and Xray crystallographic studies show that ArfR GTPases possess the mechanism of membrane binding and structural features unique to Arf family proteins. Our results indicate that Arf family GTPases originated in the archaeal ancestor of eukaryotes, consistent with aspects of the endomembrane system evolving early in eukaryogenesis. More information: https://www.nature.com/articles/s41564-024-01904-6#Abs1 Contact: Julie Ménétrey julie.menetrey@i2bc.paris-saclay.fr

|
Scooped by
I2BC Paris-Saclay
February 11, 8:55 AM
|
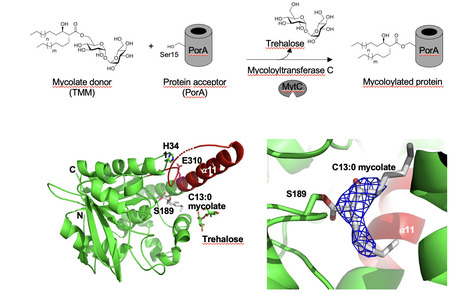
Synthetic mycolates derivatives to decipher protein mycoloylation, a unique post-translational modification in bacteria
In vitro reconstitution of bacterial protein mycoloylation. Protein mycoloylation is a newly characterized post-translational modification (PTM) specifically found in Corynebacteriales, an order of bacteria that includes numerous human pathogens. Their envelope is composed of a unique outer membrane, the so-called mycomembrane made of very-long chain fatty acids, named mycolic acids. Recently, some mycomembrane proteins including PorA have been unambiguously shown to be covalently modified with mycolic acids in the model organism Corynebacterium glutamicum by a mechanism that relies on the mycoloyltransferase MytC. This PTM represents the first example of protein O-acylation in prokaryotes and the first example of protein modification by mycolic acid. Through the design and synthesis of trehalose monomycolate (TMM) analogs, we prove that i) MytC is the mycoloyltransferase directly involved in this PTM, ii) TMM, but not trehalose dimycolate (TDM), is a suitable mycolate donor for PorA mycoloylation, iii) MytC is able to discriminate between an acyl and a mycoloyl chain in vitro unlike other trehalose mycoloyltransferases. We also solved the structure of MytC acyl-enzyme obtained with a soluble short TMM analogs which constitutes the first mycoloyltransferase structure with a covalently linked to an authentic mycolic acid moiety. These data highlight the great conformational flexibility of the active site of MytC during the reaction cycle and pave the way for a better understanding of the catalytic mechanism of all members of the mycoloyltransferase family including the essential Antigen85 enzymes in Mycobacteria. more information: https://doi.org/10.1016/j.jbc.2025.108243 Contact: Florence CONSTANTINESCO-BECKER <florence.constantinesco@i2bc.paris-saclay.fr>

|
Scooped by
I2BC Paris-Saclay
January 30, 3:50 AM
|
Genetic differentiation in the MAT-proximal region is not sufficient for suppressing recombination in Podospora anserina
A large genomic region in Podospora anserina remains entirely devoid of crossovers, despite being fully colinear. Recombination is advantageous over the long-term, as it allows efficient selection and purging deleterious mutations. Nevertheless, recombination suppression has repeatedly evolved in sex chromosomes. In fungi, sexual compatibility is driven by a locus called mating-type (mat), around which recombination suppression is also often observed.
The evolutionary causes for recombination suppression and the proximal mechanisms preventing crossing overs are poorly understood. Several hypotheses have recently been suggested based on theoretical models, and in particular, that divergence could accumulate neutrally around a sex-determining region and reduce recombination rates, a self-reinforcing process that could foster progressive extension of recombination suppression.
We used the ascomycete fungus Podospora anserina for investigating these questions: a 0.8 Mbp region around its mat locus is non-recombining (called MAT-proximal region), despite being collinear between the two mating types. This fungus is mostly selfing, resulting in highly homozygous individuals, except in the non-recombining region around the mating-type locus that displays differentiation between mating types. Here, we test the hypothesis that sequence divergence alone is responsible for recombination cessation. We replaced one mat idiomorph by the sequence of the other, to obtain compatible strains isogenic in the MAT-proximal region. Crosses showed that recombination was still suppressed in that context, indicating that other proximal mechanisms than inversions or mere sequence divergence are responsible for recombination suppression in this fungus.
This finding suggests that selective mechanisms likely acted for suppressing recombination, or the spread of epigenetic marks, as the neutral model based on mere nucleotide divergence does not seem to hold in P. anserina. More information: https://doi.org/10.1093/g3journal/jkaf015 Contact: Pierre GROGNET pierre.grognet@universite-paris-saclay.fr

|
Scooped by
I2BC Paris-Saclay
January 17, 4:17 AM
|
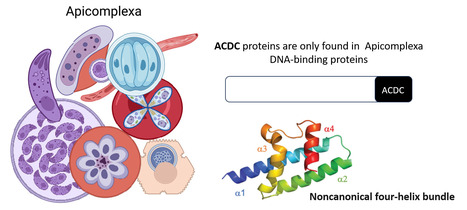
Rocking Science: New ACDC Protein Fold Unveiled
The Apicomplexa-specific ACDC domain adopts a never before seen protein fold. In collaboration with the Nessler team at the I2BC and the Llinás lab at PSU, US, we reveal that the ACDC domain, only found in DNA-binding proteins of the Apicomplexa phylum, grouping several important human pathogens including malaria, has a never before seen protein fold. We also identify potential ligands that may be optimized in the future as protein inhibitors. More information:https://journals.iucr.org/paper?S2059798324012518 Conatct: Joana SANTOS joana.santos@i2bc.paris-saclay.fr

|
Scooped by
I2BC Paris-Saclay
December 17, 2024 11:56 AM
|
DciA, the Bacterial Replicative Helicase Loader, promotes LLPS in the presence of ssDNA.
This study shows that DciA, the bacterial replicative helicase loader, promotes condensates in the presence of DNA. This opens the way to the possibility of non-membrane compartments in bacteria. The goal could be to concentrate the players involved in replication and thus facilitate it. The loading of the bacterial replicative helicase DnaB is an essential step for genome replication and depends on the assistance of accessory proteins. Several of these proteins have been identified across the bacterial phyla. DciA is the most common loading protein in bacteria, yet the one whose mechanism is the least understood. We have previously shown that DciA from Vibrio cholerae is composed of a globular domain followed by an unfolded extension and demonstrated its strong affinity for DNA. Here, drawing on the skills of two I2BC facilities, Light microscopy (Imagerie-Gif) and PIM (Structural biology), we characterize the condensates formed by VcDciA upon interaction with a short single-stranded DNA substrate. We demonstrate the fluidity of these condensates using light microscopy and address their network organization through electron microscopy, thereby bridging events to conclude on a liquid-liquid phase separation behavior. Additionally, we observe the recruitment of DnaB in the droplets, concomitant with the release of DciA. We show that the well-known helicase loader DnaC from Escherichia coli is also competent to form these phase-separated condensates in the presence of ssDNA. Our phenomenological data are still preliminary as regards the existence of these condensates in vivo, but open the way for exploring the potential involvement of DciA in the formation of non-membrane compartments within the bacterium to facilitate the assembly of replication players on chromosomal DNA. More information : https://pubmed.ncbi.nlm.nih.gov/39603490/ Contact : Sophie CHERUEL sophie.quevillon-cheruel@i2bc.paris-saclay.fr and Stéphanie MARSIN stephanie.marsin@i2bc.paris-saclay.fr

|
Scooped by
I2BC Paris-Saclay
December 9, 2024 3:44 AM
|
Ribosome composition plays a key role in EMT
Ribosome remodeling drives cancer cell transformation: A single protein, RPL36A, triggers EMT. Epithelial-mesenchymal transition (EMT) is a biological process whereby a cell loses its usual characteristics to acquire properties allowing it to move. EMT is involved in embryo formation and tissue repair in adults. However, when altered, EMT contributes to the formation of fibrosis or, in the case of a tumor cell, to the formation of metastases. In cancers, this change is comparable to a cellular metamorphosis that allows cancer cells to become more aggressive and resistant to treatments. EMT is therefore a key player in tumor progression.
EMT is extensively studied at the molecular and cellular levels because a better understanding of this process will enable the development of new therapeutic approaches. However, the translational changes that occur during EMT have so far been very poorly studied.
In this work, we have identified that the overexpression of a single ribosomal protein, called RPL36A, is sufficient to induce EMT. We have also highlighted the various translational changes that occur during EMT.
Our work reveals the importance of ribosome remodeling itself in finely modulating translation during the change in cellular identity that occurs during EMT. More information : https://www.pnas.org/doi/10.1073/pnas.2408114121 ; In French: https://www.insb.cnrs.fr/fr/cnrsinfo/des-modifications-de-la-composition-du-ribosome-accompagnent-les-changements. Contact: Olivier NAMY olivier.namy@i2bc.paris-saclay.fr
|

|
Scooped by
I2BC Paris-Saclay
April 15, 8:44 AM
|
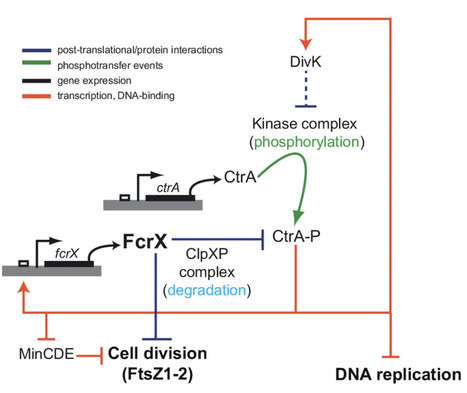
Sinorhizobium meliloti FcrX coordinates cell cycle and division during free-living growth and symbiosis by a ClpXP-dependent mechanism
In this study, a new essential player of cell cycle regulation has been characterized in the nitrogen fixing symbiont Sinorhizobium meliloti. During the nitrogen-fixing symbiosis between the alphaproteobacterium Sinorhizobium meliloti and the plant Medicago sativa (alfalfa), the interplay of molecular mechanisms governing cell cycle and bacteroid differentiation is a remarkable system that has still many details to be discovered. Here, we describe a bacterial cell cycle regulator, named FcrX, that controls two of the main essential players of cell cycle and bacteroid differentiation: the master regulator CtrA and the tubulin-like Z-ring component FtsZ. This essential factor is potentially participating with the degradosome complex driving the proteolysis of those two important regulators. Constitutive expression of FcrX during nodule development shows an increase of plant biomass, opening interesting paths in the amelioration of biological nitrogen fixation for a sustainable agriculture. More information : https://doi.org/10.1073/pnas.2412367122 Contact : Emanuele Biondi - emanuele.biondi@i2bc.paris-saclay.fr

|
Scooped by
I2BC Paris-Saclay
April 14, 3:19 AM
|
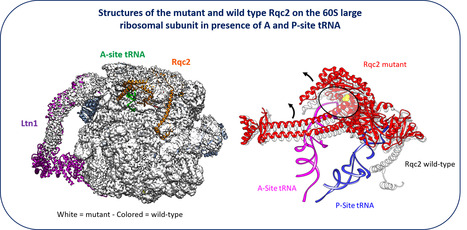
New facet of the Rqc2 protein part of a ribosome rescue pathway in Saccharomyces cerevisiae
The Ribosome Quality Complex protein 2 is a major player in peptide release from stalled ribosomes. Cells employ many quality control mechanisms during protein biosynthesis. Defects impairing messenger RNA decoding can cause ribosomes to stall, thereby distorting gene expression. Ribosome stalling results in ribosomes being sequestered in an inactive state on mRNA and represents a challenge for both ribosome homeostasis and cell survival. Eukaryotic cells prevent the accumulation of potentially toxic aberrant polypeptides and maintain ribosome availability by employing surveillance and clearance mechanisms, including the evolutionarily conserved ribosome-associated quality control complex (RQC). The rescue pathways dissociate the stalled ribosome, leading to the release of a 40S subunit attached to the mRNA and a 60S subunit still attached to a tRNA with the elongating peptide. RQC mediates the ubiquitination, extraction, and rapid degradation of the aberrant nascent peptide. In particular, Rqc2p recruits charged alanyl- and threonyl-tRNAs at the same position as an A-site tRNA, resulting in C-terminal extensions composed of alanine and threonine residues (CAT tails). These CAT tails may expose potential tunnel-embedded lysine residues for ubiquitination by the E3 ligase Ltn1. Using a genetic screen, we identified a mutant allele of RQC2 as involved in peptide release from stalled ribosomes. We characterized the mutant protein both biochemically and structurally, in collaboration with Reynald Gillet’s team (IGDR, Rennes University). We determining how the underlying point mutation reduced tRNA recruitment to the A site, thereby limiting CAT tail formation. These findings provide further mechanistic insight into the role of Rqc2p in ribosome rescue and the maintenance of ribosome homeostasis. More Information:https://pubmed.ncbi.nlm.nih.gov/40187343/ Contact: Céline Fabret celine.fabret@i2bc.paris-saclay.fr

|
Scooped by
I2BC Paris-Saclay
April 7, 5:04 AM
|
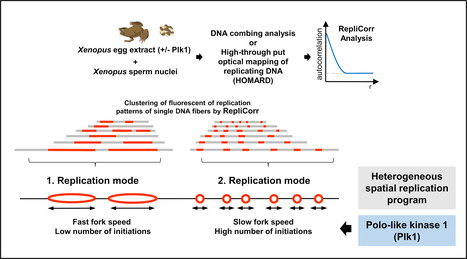
Yin-Yang during DNA replication
DNA duplication of the genetic material is a crucial step of cell proliferation. The study reveals that this step comprises two opposed strategies, coordinated by the enzyme Plk1. In vertebrates, DNA replication involves the activation of thousands of starting points for DNA copying, known as “origins of replication”. These points are irregularly scattered across the genome. However, the precise mechanisms controlling the coordinated activation of these starting points and the regulation of the rate at which DNA is copied remain poorly understood. Any dysfunction can lead to genetic abnormalities and promote the development of cancers.
The scientists studied DNA replication using cell-free extracts from the eggs of the amphibian Xenopus laevis. This model is quite similar to replication in human cells, and has made it possible to develop a new method for analyzing the distribution of replication starting points (initiations) and the speed of replication in individualized DNA molecules. Combining this analysis with kinetic models, the scientists discovered that DNA replication relies on two dynamic and opposing strategies:
A fast strategy: high replication speed, but with fewer starting points.
A slow strategy: a slower replication speed compensated by the activation of numerous starting points.
In this context, the scientists determined that the enzyme Polo-like kinase 1 (Plk1) played a key role in coordinating these two strategies. Plk1, frequently mutated in many cancers, regulates this balance, opening up new perspectives on the control of replication and its implications in oncology. (published by Paris-Saclay and CNRS Biologie Actu (https://www.insb.cnrs.fr/fr/cnrsinfo/yin-yang-dans-la-duplication-des-chromosomes ) More Information: https://doi.org/10.1093/nar/gkaf007 Contact: Arach Goldar arach.goldar@i2bc.paris-saclay.fr Kathrin Marheineke kathrin.marheineke@ijm.fr

|
Scooped by
I2BC Paris-Saclay
April 3, 6:36 AM
|
Exploring the Interaction of Human α‐Synuclein with Polyethylene Nanoplastics: Insights from Computational Modeling and Experimental Corroboration
Can the presence of microplastics in the brain affect proteins? Plastics are now part of our daily lives because they are used in so many different ways. They are therefore a major source of pollution. Their chemical stability has consequently become a major concern for the environment and health, given their presence in all ecosystems. The penetration of plastic particles into living organisms through ingestion or inhalation has now been widely demonstrated. Micro- and nanoplastics are found throughout the human body, including in the brain, which raises the question of their potential toxicity. In a biological environment, the plastic particle does not remain naked but interacts with the surrounding molecules to form a biocorona. In this study, Tripathi et al. investigate the binding behavior of human α-synuclein (hαSn) with polyethylene (PE)-based plastics using molecular dynamics simulations and experimental methods. Their simulations show that (a) hαSn folds into a compact conformation to enhance intramolecular interactions, (b) non-oxidised PE nanoplastics facilitate the rapid adsorption of hαSn onto its surface with a change in the structural properties of hαSn, and (c) oxidised nanoplastics fail to capture hαSn. The experimental dynamic light scattering and adsorption isotherms are in good agreement with simulations. The observed formation of the plastic nanoparticle complex with hαSn can be proposed as a plausible pathogenic driving force in neuronal dysfunction and subsequent neurological damage. More information : https://doi.org/10.1021/acs.biomac.4c00918 Contact: Yves Boulard yves.boulard@i2bc.paris-saclay.fr

|
Scooped by
I2BC Paris-Saclay
April 2, 8:28 AM
|
The I2BC crystallization platform is evolving into a crystallography platform, offering new services
The crystallization platform has evolved into a crystallography platform and now offers new services (including protein structure determination and/or analysis) as well as scientific support for clients in structural biology projects. Protein crystallography is the main approach for characterizing the 3D structure of proteins associated with drug candidates and for determining the molecular basis of complexes between soluble or membrane proteins and their partners (DNA, RNA, other proteins, peptides, lipids, cofactors, various ligands including metals). Initially, the crystallization platform was dedicated to automated screening, analysis, and optimization of crystallization conditions for soluble and membrane macromolecules. Now, the crystallography platform provides extended services, including protein structure determination and/or analysis. The successive steps, starting from a purified protein sample of interest, include crystal formation, diffraction image collection at the Synchrotron Soleil, electron density map calculation, atomic model refinement of the protein or complex of interest, and structural analysis. Emphasis is placed on obtaining the most accurate models for the most successful structural analysis. Additionally, an AlphaFold service, including predicted model analysis, is available, relying on services established at I2BC by the BIOI2 integrative bioinformatics platform. Routine upstream steps before obtaining a protein crystal include obtaining an optimized synthetic gene for expression in prokaryotic hosts, expression, and purification of the recombinant protein of interest. The platform can advise clients on these various steps, and collaboration or service provision can be considered depending on project complexity. The crystallography platform is open to all scientists, from beginners to experienced crystallographers. It is important to note that the quality of the protein sample is a crucial criterion. The sample must be pure and verified on a denaturing acrylamide gel before crystallization. Do not hesitate to contact us. Contact: cristallographie@i2bc.paris-saclay.fr
More information: https://www.i2bc.paris-saclay.fr/structural-biology/crystallography/

|
Scooped by
I2BC Paris-Saclay
March 4, 3:20 AM
|
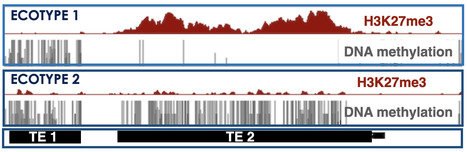
Alternative silencing states of transposable elements in Arabidopsis associated with H3K27me3
This study sheds light on an alternative mode of Transposable Element silencing associated with H3K27me3 instead of DNA methylation in flowering plants; it also indicates dynamic switching between the two epigenetic marks at the species level, a new paradigm that might extend to other multicellular eukaryotes. The DNA/H3K9 methylation and Polycomb-group proteins (PcG)-H3K27me3 silencing pathways have long been considered mutually exclusive and specific to transposable elements (TEs) and genes, respectively in mammals, plants, and fungi. However, H3K27me3 can be recruited to many TEs in the absence of DNA/H3K9 methylation machinery and sometimes also co-occur with DNA methylation.
In this study, the team of Angélique Déléris showed that TEs can also be solely targeted and silenced by H3K27me3 in wild-type Arabidopsis plants. These H3K27me3-marked TEs not only comprise degenerated relics but also seemingly intact copies that display the epigenetic features of responsive PcG target genes as well as an active H3K27me3 regulation. The authors also showed that H3K27me3 can be deposited on newly inserted transgenic TE sequences in a TE-specific manner indicating that silencing is determined in cis. Finally, a comparison of Arabidopsis natural accessions reveals the existence of a category of TEs—which we refer to as “bifrons”—that are marked by DNA methylation or H3K27me3 depending on the accession. This variation can be linked to intrinsic TE features and to trans-acting factors and reveals a change in epigenetic status across the TE lifespan.
Overall, this study shedded light on an alternative mode of TE silencing associated with H3K27me3 instead of DNA methylation in flowering plants. It also suggests dynamic switching between the two epigenetic marks at the species level, a new paradigm that might extend to other multicellular eukaryotes. More Information: https://genomebiology.biomedcentral.com/articles/10.1186/s13059-024-03466-6 Contact: Angélique Déléris angelique.deleris@i2bc.apris-saclay.fr

|
Scooped by
I2BC Paris-Saclay
February 17, 5:00 AM
|
ChemPhysBio2025 : Registrations Now Open!
We’re excited to announce that registrations are now open for the Second Interdisciplinary Summer School: ChemPhysBio2025 – Imaging Biology: Interdisciplinary Tools and Techniques to Unravel Cellular Dynamics – taking place June 16–20, 2025, at Université Paris-Saclay! This unique event bridges chemistry, physics, and biology, offering participants the opportunity to explore advanced photonic imaging techniques for unraveling cellular dynamics. We’re also thrilled to welcome two distinguished guest lecturers: - Julie Karpenko (Laboratory for Therapeutic Innovation, University of Strasbourg)
- Balázs Enyedi (Department of Physiology, Semmelweis University, Hungary)
What to expect? - Inspiring lectures and research seminars
- Interactive interdisciplinary workshops
- Hands-on experience in cutting-edge research labs
Who should apply?
Master’s students, PhD candidates, postdocs, and junior researchers who are newcomers to the interdisciplinary field and eager to deepen their knowledge, expand their skill sets, and build meaningful collaborations. With only 20 spots available, don’t miss your chance to join us!
Apply now: https://chemphysbio2025.sciencesconf.org/

|
Scooped by
I2BC Paris-Saclay
February 11, 9:48 AM
|
Complete description of the biosynthetic process of [2Fe-2S] clusters
Iron-sulfur clusters are essential metallocofactors providing catalytic activities to a multitude of enzymes and proteins. Here, we report a comprehensive picture of their assembly mechanism, showing that it relies on the formation of [1Fe-1S] precursors fused into [2Fe-2S] clusters upon dimerization of the scaffold protein. Iron-sulfur (Fe-S) clusters are ubiquitous metallocofactors constituting the active site of a multitude of enzymes and proteins involved in electron transfer, catalysis, sulfur donation and signalling. They are made of iron and sulfide ions assembled into diverse structures. The [2Fe-2S] and [4Fe-4S] clusters are the most common forms in organisms. They are synthesized by multi-protein machineries which have remained highly conserved during evolution. The iron-sulfur cluster (ISC) assembly machinery present in eukaryotes and prokaryotes synthesizes [2Fe-2S] clusters, which serve as building blocks for the assembly of [4Fe-4S] clusters. The core ISC machinery assembles [2Fe-2S] clusters on the scaffold protein IscU, which requires iron provided by an unknown source, sulfur provided in the form of cysteine bound persulfides (Cys-SSH) by the cysteine desulfurase IscS, and electrons provided by the ferredoxin–ferredoxin reductase complex Fdx-FdxR from NADPH. Then, specialized chaperones transfer [2Fe-2S] clusters to recipient acceptors. Despite previous studies on the core assembly machinery, the mechanistic details of the [2Fe-2S] cluster assembly process have remained poorly understood due to the experimental difficulties in trapping the relevant intermediates.
The team Biochemistry of Metalloproteins and Associated Diseases at the Institute of Integrative Biology of the Cell in Gif-Sur-Yvette (I2BC, UMR 9198, CNRS – CEA - Paris-Saclay University) managed to dissect this process step by step and to isolate several key intermediates using a functional reconstitution of the Escherichia coli ISC machinery. They used a combination of biochemical techniques to trap these intermediates: anaerobic reconstitution, persulfide detection assays, kinetics, UV-visible, circular dichroism, and to characterize them by spectroscopic methods: electron paramagnetic spectroscopy (EPR), nuclear magnetic resonance (NMR) and native mass spectrometry (nMS) in collaboration with teams at Aix-Marseille University (BIP), Gif-Sur-Yvette (ICSN) and Strasbourg (IPHC). They show that the assembly of [2Fe-2S] clusters is initiated by iron binding to IscU, which triggers persulfide insertion by IscS in the vicinity of the iron-binding site of IscU upon the formation of a complex between IscU and IscS. The persulfide in IscU binds to the iron center and is cleaved into sulfide by the Fdx-FdxR complex, which leads to the formation of a Fe-SH intermediate, referred to as the [1Fe-1S] precursor. Then, IscU dissociates from IscS, dimerizes and generates a bridging [2Fe-2S] cluster by fusion of two [1Fe-1S] precursors. The IscU dimer ultimately dissociates into a monomer, ready to transfer its [2Fe-2S] cluster to acceptors. The data also indicate that the bridging cluster is initially in the super-reduced state [2Fe-2S]0 and releases two electrons to the ferredoxin enzyme, thereby leading to an oxidised [2Fe-2S]2+ state as the final product.
These data provide a comprehensive description of mechanism of [2Fe-2S] clusters assembly by the bacterial ISC machinery, highlighting the formation of key intermediates through a tightly concerted process. This stepwise dissection further supports findings in eukaryotes, including iron loading, persulfidation and dimerization of IscU, which point to an evolutionary conservation of the assembly process. More information: https://www.nature.com/articles/s41589-024-01818-8 Contact: Benoit D'Autréaux benoit.dautreaux@i2bc.paris-saclay.fr

|
Scooped by
I2BC Paris-Saclay
February 10, 9:09 AM
|
Tutorial videos for ChimeraX

|
Scooped by
I2BC Paris-Saclay
January 21, 9:18 AM
|
The Biology and Physics of Prokaryotic Chromosomes VII
The Biology and Physics of Prokaryotic Chromosome is an international scientific conference, bringing together experimentalists and theoreticians to discuss discoveries and challenges around building an integrated model of prokaryotic chromosome structure. The conference focuses on mechanisms of chromosome organisation and structure, chromosome replication, transcription, and segregation, investigated at multiple scale lengths, using both experimental and theoretical approaches. A key goal is to provide a platform for inter-disciplinary work, to foster new collaboration and develop new ideas. New approaches and technologies have revolutionised the field of prokaryotic chromosome biology in the last fifteen years. For example, super-resolution microscopy is now being used to visualize previously unresolved structure, genomic tools have enabled the capture of new types of proximity information on a genomic-scale, and polymer physics models allow researchers to tackle questions that go far beyond the traditional biological description of chromosome structure. Join us in York for an unforgettable scientific meeting - this is an opportunity you won’t want to miss. At least half of the oral presentation slots are expected to be selected from submitted abstracts and we will prioritise early career researchers. Programme Coordinators:
David Grainger, University of Birmingham
Daniela Barillà, University of York
Remus Dame, Leiden University
Virginia Lioy, Institute for Integrative Biology of the Cell More information: bit.ly/bio-prokaryotic-VII Contact: Virginia LIOY virginia.lioy@i2bc.paris-saclay.fr

|
Scooped by
I2BC Paris-Saclay
January 16, 8:23 AM
|
Newcomer at the BIOI2 bioinformatics platform
Baptiste Roelens joined the BIOI2 platform last month, bringing expertise in machine learning for biological data analysis. His contributions will expand the platform's service offerings and support the I2BC imaging community. The integrative BIOinformatics platform (BIOI2) welcomes Baptiste Roelens as a new engineer. Baptiste's background combines theoretical computer science and life sciences, with a foundation from Ecole Polytechnique. He further honed his skills at the Institut Curie, where he earned his PhD. His doctoral research focused on developing quantitative approaches to analyze the role of the histone chaperone CAF-1 in maintaining higher-order chromatin structure during meiotic prophase in the fruit fly. Continuing his research journey, Baptiste joined Stanford University to develop bioimage analysis pipelines, further exploring the mechanisms regulating chromosome organization and cell-cycle progression in C. elegans germline. Upon returning to France, he contributed to the biotech company Viroxis, developing cutting-edge deep-learning approaches for protein design. With experience spanning both wet and dry labs, Baptiste will be a great asset to the BIOI2 platform and is ideally placed to collaborate with the I2BC’s imaging community, in particular the Imagerie-Gif platform (https://www.i2bc.paris-saclay.fr/bioimaging/), to develop pipelines that streamline the processing and analysis of complex imaging data. BIOI2 provides access to bioinformatics resources and activities developed and of use at the I2BC, organises focused training session on bioinformatics tools, and is an official contributing plateform of the nationwide French Institute of Bioinformatics (IFB). More information: https://bioi2.i2bc.paris-saclay.fr Contact: contact-bioi2@i2bc.paris-saclay.fr

|
Scooped by
I2BC Paris-Saclay
December 16, 2024 5:49 AM
|
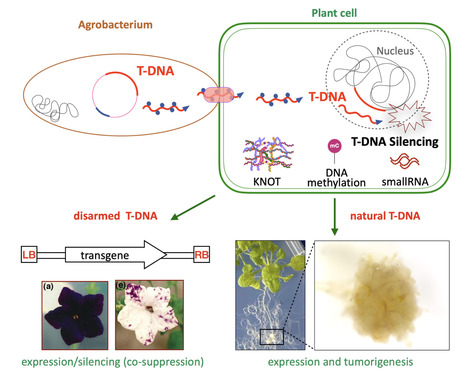
Epigenetic control of T-DNA during transgenesis and pathogenesis
T-DNAs are mobile elements transferred from pathogenic Agrobacterium to plants that reprogram host cells into hairy roots or tumors, and which are used as disarmed forms to deliver transgenes in plants. Here we review the mechanisms that silence the expression of T-DNAs in transgenic plants as well as during pathogenesis. Mobile elements known as T-DNAs are transferred from pathogenic Agrobacterium to plants and reprogram the host cell to form hairy roots or tumors. Disarmed nononcogenic T-DNAs are extensively used to deliver transgenes in plant genetic engineering. Such T-DNAs were the first known targets of RNA silencing mechanisms, which detect foreign RNA in plant cells and produce small RNAs that induce transcript degradation. These T-DNAs can also be transcriptionally silenced by the deposition of epigenetic marks such as DNA methylation and the dimethylation of lysine 9 (H3K9me2) in plants. Here, we review the targeting and the roles of RNA silencing and DNA methylation on T-DNAs in transgenic plants as well as during pathogenesis. In addition, we discuss the crosstalk between T-DNAs and genome-wide changes in DNA methylation during pathogenesis. We also cover recently discovered regulatory phenomena, such as T-DNA suppression and RNA silencing-independent and epigenetic-independent mechanisms that can silence T-DNAs. Finally, we discuss the implications of findings on T-DNA silencing for the improvement of plant genetic engineering. More information: https://academic-oup-com.insb.bib.cnrs.fr/plphys/advance-article/doi/10.1093/plphys/kiae583/7876130 Contact: Angélique DELERIS angelique.deleris@i2bc.paris-saclay.fr
|





 Your new post is loading...
Your new post is loading...









![Complete description of the biosynthetic process of [2Fe-2S] clusters | I2BC Paris-Saclay | Scoop.it](https://img.scoop.it/THKpAN1-eQiW-1DEJF_ZmDl72eJkfbmt4t8yenImKBVvK0kTmF0xjctABnaLJIm9)