Your new post is loading...
 Your new post is loading...

|
Scooped by
Gilbert C FAURE
March 25, 2020 4:03 PM
|
In the era of precision oncology, liquid biopsy techniques, especially the use of plasma circulating tumour DNA (ctDNA) analysis, represent a paradigm shift in the use of genomic biomarkers with considerable implications for clinical practice. Compared with tissue-based tumour DNA analysis, plasma ctDNA is more convenient to test, more readily accessible, faster to obtain and less invasive, minimizing procedure-related risks and offering the opportunity to perform serial monitoring. Additionally, genomic profiles of ctDNA have been shown to reflect tumour heterogeneity, which has important implications for the identification of resistant clones and selection of targeted therapy well before clinical and radiographic changes occur. Moreover, plasma ctDNA testing can also be applied to cancer screening, risk stratification and quantification of minimal residual disease. These features provide an unprecedented opportunity for early treatment of patients, improving the chances of treatment success. Liquid biopsy techniques, especially the use of plasma circulating tumour DNA (ctDNA) analysis, are a convenient, fast and non-invasive approach to the diagnosis and monitoring of urological cancers, and could enable selection of targeted therapy before clinical and radiographic changes occur. In this Review, the authors discuss the uses of liquid biopsy and plasma ctDNA analysis in particular, and consider how it could be used in clinical practice now and in the future.

|
Scooped by
Gilbert C FAURE
November 23, 2019 4:08 AM
|
Cancer originates from small mistakes in our DNA. These mistakes are different for every person and dictate how aggressive their cancer is.On top of this, starting in development and continuing throughout life, new mistakes in our DNA can be …...

|
Scooped by
Gilbert C FAURE
August 18, 2019 2:21 PM
|
Room for improvement exists regarding recommendations for screening, staging, therapy selection, and frequency of surveillance of gastrointestinal cancers. Screening is costly and invasive, improved staging demands increased sensitivity and specificity to better guide therapy selection.

|
Scooped by
Gilbert C FAURE
August 2, 2019 4:44 AM
|
We perform single-cell genomic profiling of CTCs and pair the genomics to digital pathology features allowing us to see both cell phenotype and genotype

|
Scooped by
Gilbert C FAURE
June 18, 2019 6:46 AM
|
The Lund, Sweden-based company raised $4.1 million to accelerate commercialization of its portfolio of kits and services.

|
Scooped by
Gilbert C FAURE
May 8, 2019 2:31 PM
|
With the combined advantages of optical microscopy and flow cytometry, imaging flow cytometry (IFC) has quickly become an established tool for performing cytometric analysis in diverse areas of biology including microbiology, immunology, and stem cell biology1-14. IFC provides quantitative, spatially registered image data for every event analyzed, which allows the morphometric characterization of single cells in large heterogeneous populations, further advancing our understanding of cell‐to‐cell differences in physiological settings. The availability of image data produced by IFC is well aligned with the pressing need for progressively larger biomedical datasets for efficient and accurate data analysis with the help of computational tools (e.g., compressive imaging, data mining, machine/deep learning) to make better decisions in biomedical research and clinical settings. Recent studies 4, 5, 10, 12 show that IFC is highly effective for the characterization of DNA damage and repair, the accurate assessment of cell death and autophagy, fluorescence in situ hybridization (FISH), the localization and enumeration of specific molecules such as proteins, nucleic acids, and peptides, and the analysis of cell–cell interaction and cell cycle. Furthermore, a number of potential clinical applications of IFC are conceivable, such as liquid biopsy, leukemia detection, and identification of infectious diseases in which the availability of high‐content single‐cell images plays an important role. Objective and Highlights of the Special Issue Building on the excellent capabilities of IFC, the objective of this Special Issue is to provide a valuable forum where scientists, engineers, and users in the field share their most recent findings and understanding of basic principles, advanced techniques, and applications of IFC. Topics covered can be broadly categorized into (1) advanced imaging methods for IFC, (2) biological and clinical applications of IFC, and (3) computational techniques for IFC. Specifically, the first part of the Special Issue consists of nine papers (while the second part follows in a subsequent issue). Dey et al. 15 report the use of IFC to accurately differentiate between active and viable but not‐culturable forms of Psedomonas aeruginosa (a Gram‐negative bacterium abundant in the environment and water systems). Kim et al. 16 present their investigation of the physical and magnetic properties of monocytes in human blood together with plasma platelets, oxyhemoglobin red blood cells, and methemoglobin red blood cells by using IFC. Lelliott et al. 17 describe their development of an automated analysis method to rapidly evaluate cells that form neutrophil extracellular traps or precursors to neutrophil extracellular traps and differentiate them from cells that undergo other forms of cell death. Zhang et al. 18 report their development of a computational method for intelligent de‐blurring of out‐of‐focus cell images in IFC by deep learning. Gu et al. 19 present their demonstration of image‐guided cell sorting together with a cell classification system and real‐time isolation of single cells based on nuclear localization of glucocorticoid receptor, particle binding to the cell membrane, and DNA damage induced γ‐H2AX foci using the instrument. Goktas et al. 20 report a combination of IFC and angle‐resolved light scattering measurements to study the morphological features of red blood cells in sickle cell patients. Hui et al. 21 describe their development of a method, which they refer to as immuno‐flowFISH, for conducting FISH on immunophenotyped whole cells in suspension to detect chromosomal abnormalities in chronic lymphocytic leukemia. Lee et al. 22 report their development of a quantitative phase IFC system to conduct large‐scale biophysical phenotyping of numerous single cells (>1,000,000 cells) in a label‐free manner. Yaakov et al. 23 report their quantitative characterization of the kinetics of Mimivirus infection stages by IFC. History The field of IFC dates back to 1979 when Kay et al. 1 and Kachel et al. 2 first introduced and experimentally demonstrated imaging in flow. Since then, the field of IFC has slowly gained interest from the research community over the past two decades (Fig. 1). Before the year 2002, only a handful of researchers with interdisciplinary expertise in optics (in particular, optical imaging), fluidics, electronics, and cell biology were capable of building IFC‐capable systems and conducting experiments while many other researchers could appreciate the great potential of the technology. This situation dramatically changed around 2008 when the Amnis Corporation (now Luminex Corporation) successfully launched the first commercial IFC system. This has helped to democratize IFC from only a few specialist research groups at academic institutions to place IFC in the hands of many researchers worldwide, as shown by the sharp rise in the number of publications in Figure 1. As of today, IFC systems have been installed in research laboratories and flow cytometry core facilities at a number of universities and other research institutions to tackle problems unanswered by conventional (non‐imaging) flow cytometry. Future Perspective The field of IFC is expected to grow further in the next decade or so (Fig. 2). There are several driving factors that push the frontier of IFC and expand its utility in diverse areas of biology and medicine. The first driving factor is the emergence of advanced microfluidics and its integration into IFC platforms 7, 8, 12-14. Microfluidics provides higher degrees of freedom in multiplexing, automation, and parallelization than traditional capillary‐based fluidics in flow cytometry, thereby adding more functionalities for preparation, analysis, and manipulation of cells. The second driving factor is the advent of various optical imaging modalities other than traditional image acquisition based on CCD image sensors with time‐delay integration 10. They include time‐stretch imaging 8, 13, frequency‐division‐multiplexed imaging 9, 24, spatial–temporal transformation 14, temporally coded excitation 25, and stroboscopic fluorescence imaging 6, which are designed to handle sensitive image acquisition of fast‐flowing cells. The third driving factor is the availability of sophisticated digital image processing techniques, such as compressive imaging, data mining, and deep learning, powered by the rapidly evolving smartphone industry 4, 5, 7, 18, 19. The big data brought by IFC systems acts as a fuel for artificial neural networks that can characterize and classify cells with higher accuracy than classical image analysis algorithms. The fourth driving factor is the advent of image‐activated cell sorting (IACS) (also called image‐guided cell sorting) 7, 19, a technology that achieves the best of both worlds between IFC and fluorescence‐activated cell sorting (FACS). IACS extends the capabilities of FACS from one‐dimensional signal intensities to multidimensional information‐rich images, which can be used to analyze the spatial architecture of single cells. IACS offers the opportunity to study and exploit sorted cells via single‐cell analysis tools such as genomics, proteomics, and proteomics. With these driving factors, the number of researchers, applications, discoveries, and publications in the field of IFC is expected to go up in the next decade or so (Fig. 2). The future of IFC is bright. Literature Cited

|
Scooped by
Gilbert C FAURE
April 9, 2019 2:37 PM
|
Dr. David Wong, associate dean for research, and his team received regulatory approval on their EFIRM (electric field-induced release and measurement) technology as a Clinical Laboratory Improvement Amendments (CLIA)-certified, College of American Pathology (CAP)-accredited assay to be used in clinical practice. EFIRM creates a reliable, novel, and impactful method to detect tumor-causing, lung cancer mutations in saliva and blood that is non-invasive, cost-effective, and quick. For the past four years until now, the technology has been in research and testing phases. Small celebration after the technology received its regulatory approval. Left to right: Drs. David Wong, Charles Strom, Michael Tu, Paul Krebsbach (Dean), Richard Bender, and Josh Deignan. A major contributor to helping move the EFIRM testing phase along is the National Cancer Institute (NCI), who awarded Dr. Wong and his collaborators, Drs. Wayne Grody and Josh Deignan with the UCLA Department of Pathology and Laboratory Medicine and Drs. Fang Wei, Charles Strom and Michael Tu of the School of Dentistry, $2.5M in 2017. The grant’s support successfully paid off, as the technology is now approved to be used in medical offices to assist a doctor’s diagnosis of a major disease—lung cancer. The goal is to use results from EFIRM, based on a patient’s saliva sample, to adjust therapeutic strategies in real-time, improving clinical outcomes. Another grant from by the National Cancer Institute, awarded in October 2018 for $5M over five years, is continuing to develop and refine the technology for early assessment of cancer. Dr. Wong’s lab is working with Dr. Denise Aberle, professor of radiology at the David Geffen School of Medicine at UCLA, Dr. Steven Dubinett, co-investigator and chief of pulmonary and critical care medicine in the Geffen School, and We Liao, co-inventor of the technology, to test the blood and saliva of at least 300 at-risk patients for lung cancer. Dr. Wong’s team has shown that the technology can detect early-stage lung cancer with greater than 90 percent similarity with traditional tissue biopsies. In another defining moment for liquid biopsy and salivary diagnostics, Dr. Wong was invited to speak on the EFIRM Liquid Biopsy technology in an NCI-sponsored session at the annual meeting for the American Association for Cancer Research (AACR) in Atlanta, Georgia on April 2. In front of hundreds of cancer researchers from around the world, Wong was included in a session on the topic; “Liquid Biopsy: The Holy Grail for Early Cancer Detection.” Dr. Wong also chairs the Steering Committee of the NCI Liquid Biopsy consortium. Research panel at AACR, from left to right: Drs. Sudhir Srivastava (Chief NCI, Early Detection Research Network (EDRN), Lynn Sorbara (NCI Program Director), David Wong (UCLA), Bob Carter (Harvard/MGH), Changhuei Yang (CALTech), Nicholas Papadopoulos (Johns Hopkins). "Receiving regulatory approval for the EFIRM technology and also having the opportunity to present our research findings at a major cancer research meeting proves that salivary diagnostics must be integrated with mainstream, cutting-edge cancer early detection, screening and risk assessment,” Dr. Wong said.

|
Scooped by
Gilbert C FAURE
February 22, 2019 9:26 AM
|
In this Review, Pantel and Alix-Panabières provide an overview of approaches for the detection and characterization of minimal residual disease (MRD) using circulating tumour cells and circulating tumour DNA. They also discuss the clinical implications of such liquid biopsy approaches to MRD monitoring for the management of patients with cancer.

|
Scooped by
Gilbert C FAURE
January 9, 2019 8:50 AM
|
The way we think about cancer is about to change. What we used to treat as a random occurrence is becoming both predictable and preventable through an array of emerging circular biomarkers. Through the use of circulating tumor cells, cell-free circulating DNA, exosomes, exomers, oncosomes and other extracellular vesicles, we are now able to understand early stages of cancer and apply molecular profiling to clinical trial selection, patient treatment and minimal residual disease detection. The applications are expanding outside of cancer to include transplant medicine, cardiovascular disease, CNS, autoimmune and infectious disease. Encounter the latest research results and clinical trial data while networking with leaders in the field. Final Agenda Day 1 | Day 2 | Day 3 | Download Brochure Scientific Advisory Board Stefanie Jeffrey, MD, John and Marva Warnock Professor, Surgery; Chief, Surgical Oncology Research, Stanford University School of Medicine Stuart S. Martin, PhD, Professor, Physiology, Marlene and Stewart Greenebaum NCI Comprehensive Cancer Center, University of Maryland School of Medicine Catherine Alix-Panabières, PhD, Director, Laboratory of Rare Human Circulating Cells (LCCRH), Pathology and Onco-Biology Department, University Medical Center of Montpellier Klaus Pantel, MD, Professor and Founding Director, Institute of Tumor Biology, University, Medical Center Hamburg-Eppendorf Steven A. Soper, PhD, Professor, Micro and Nanofabricated Tools for Biological Discovery and Medical Diagnostics, University of Kansas Shannon L. Stott, PhD, Assistant Professor, Department of Medicine, Massachusetts General Hospital, Harvard Medical School; BioMEMS Resource Center, The MGH Cancer Center Arrive Early for: SUNDAY, MARCH 10, 2:00 - 5:00 PM (AFTERNOON SHORT COURSES) SC4: Translating CTCs and ctDNA for Clinical Use - Detailed Agenda SUNDAY, MARCH 10, 5:30 - 8:30 PM (DINNER SHORT COURSES) SC14: Liquid Biopsy Technologies and Applications - Detailed Agenda MONDAY, MARCH 11, 8:00 - 11:00 AM (MORNING SHORT COURSES) SC21: Detection and Characterization of Circulating Biomarkers - Detailed Agenda MONDAY, MARCH 11 10:30 am Conference Program Registration Open OPENING KEYNOTE SESSION: WHAT NEEDS TO BE DONE TO BRING CTCS TO THE CLINIC? NEXT STUDIES 11:50 Chairperson’s Opening Remarks Klaus Pantel, MD, Professor and Founding Director, Institute of Tumor Biology, University, Medical Center Hamburg-Eppendorf 12:00 pm Circulating Tumor Cells in Breast Cancers: Current Clinical Validity and Utility François-Clément Bidard, MD, PhD, Professor of Medical Oncology, Institut Curie & Versailles St. Quentin University Numerous clinical data have been collected regarding the clinical validity of CTC count and characterization in both early and advanced breast cancer patients. After having reviewed these data, we will discuss how CTC detection may help customize breast cancer therapy. 12:20 Critical Assessment of the Challenges of Using Blood-Based Biomarkers in Prostate Cancer from a Clinical Point of View Daniel C. Danila, MD, Medical Oncology Fellow, Genitourinary Oncology Service, Department of Medicine, Memorial Sloan Kettering Cancer Center In addition to determining whether circulating tumor cells (CTC) can be used as prognostic biomarkers for patients needing further therapy, current studies have proposed many predictive and pharmacodynamic biomarkers to facilitate the doctor’s ability to optimize and adjust dosage based on their ability to target specific pathways. Significant efforts have been focused on developing and analytically validating predictive biomarkers to select patients most likely to benefit the specific therapy. 12:40 PANEL DISCUSSION 1:00 Session Break 1:10 Luncheon Presentation I to be Announced 1:40 Luncheon Presentation II to be Announced 2:10 Session Break NUCLEIC ACID ANALYSIS IN EXTRACELLULAR VESICLES AND CTCS 2:30 Chairperson’s Remarks Klaus Pantel, MD, Professor and Founding Director, Institute of Tumor Biology, University, Medical Center Hamburg-Eppendorf 2:40 The Whole Transcriptional Landscape of Circulating Tumors Cells in Metastatic Breast Cancer Julie E. Lang, MD, Associate Professor of Clinical Surgery; Director, Breast Cancer Program, Department of Surgery, Keck School of Medicine, University of Southern California The aim of our study was to evaluate if RNA Seq of circulating tumor cells could serve as a surrogate for biopses of macrometastases. We evaluated treatment opportunities based on circulating tumor cell profiles and tracked them under the selection pressure of systemic therapy in Stage IV breast cancer. RNA Seq of circulating tumor cells may be used to discover molecular alterations that are potentially clinically actionable. 3:10 Large Oncosomes as a Source of Cancer-Specific Circulating Biomarkers Dolores Di Vizio, MD, PhD, Division of Cancer Biology and Therapeutics, Departments of Surgery, Biomedical Sciences and Pathology and Laboratory Medicine, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center Extracellular vesicles (EVs) are heterogeneous membrane enclosed structures harboring molecular cargo from the cell of origin. Large oncosomes are a novel subtype of EV that is released by highly migratory cancer cells. Large oncosomes contain abundant tumor-derived cargo, which can be analyzed upon purification from plasma and other biological fluids. Here we show analyses of protein, RNA and DNA in controlled system and patient plasma. 3:40 Molecular Analysis of Circulating Tumor Cells, Extracellular Vesicles and Nucleic Acids as Liquid Biopsy in the Follow-Up of Metastatic Breast Cancer Patients to Stratify Patients for Targeted Therapy Prof. Dr. Sabine Kasimir-Bauer, Head of Research Laboratory, Department of Gynecology and Obstetrics, University Hospital Essen The so-called liquid biopsy is discussed to be a surrogate marker for therapy stratification of metastatic breast cancer patients. We compared and analyzed RNA profiles enclosed in circulating tumor cells or extracellular vesicles and performed mutational analysis of cell-free DNA (cfDNA) in plasma samples (Next Generation Sequencing) in the follow-up of the disease to get insights into their feasibility for therapy stratification and to predict therapeutic options. 4:10 An Open, End to End, and Flexible Platform for Scalable CTC Collection, Identification, and Analysis Tad George, PhD, Senior Director of Platform, Applications, RareCyte RareCyte provides instrumentation and consumables that enable an exquisitely sensitive, accurate, simple, and repeatable workflow from liquid biopsy to single cell isolation. The open and end to end RareCyte CTC workflow will be the focus of this talk. 4:40 Refreshment Break and Transition to Plenary Session 5:00 Plenary Keynote Session 6:00 Grand Opening Reception in the Exhibit Hall with Poster Viewing 7:30 Close of Day Day 1 | Day 2 | Day 3 | Download Brochure TUESDAY, MARCH 12 7:30 am Registration Open and Morning Coffee 8:00 Plenary Keynote Session 9:15 Refreshment Break in the Exhibit Hall with Poster Viewing CTCS AND LIQUID BIOPSY – NEW WINDOW INTO SELECTING BEST TREATMENT FOR PATIENTS 10:15 Chairperson’s Remarks Stuart S. Martin, PhD, Professor, Physiology, Marlene and Stewart Greenebaum NCI Comprehensive Cancer Center, University of Maryland School of Medicine 10:25 Chemotherapy-Induced Pro-Metastatic Changes in Breast Cancer Microenvironment Maja H. Oktay, MD, PhD, Professor of Pathology, The L.G. Koss Division of Cytology, Montefiore Medical Center; Professor of Anatomy and Structural Biology; Director, Clinical Imaging Applications, Integrated Imaging Program, Michael F. Price Center for Genetic and Translational Medicine, Albert Einstein College of Medicine Chemotherapy may induce pro-metastatic changes within breast cancer microenvironment in both mouse and human breast cancer and subsequent hematogenous cancer cell dissemination to distant sites. Cellular and molecular mechanism involved in chemotherapy-mediated cancer cell dissemination as well as pharmacological inhibitors of this process will be discussed. 10:55 RNA Signatures in CTCs for Precision Cancer Medicine David T. Miyamoto, MD, PhD, Assistant Professor, Radiation Oncology, Harvard Medical School; Department of Radiation Oncology, Center for Cancer Research, Massachusetts General Hospital CTC analysis is a type of liquid biopsy that enables RNA expression profiling, an analysis not possible with ctDNA. Microfluidic enrichment of CTCs followed by RNA-based digital PCR enables the high-throughput and highly specific detection of CTC molecular signatures in several cancers. These CTC signatures can predict therapeutic responses and may enable the early detection of invasive cancers, thus guiding the precision management of cancer patients. 11:25 Assessing PD-L1 Expression on CTCs and Correlation with Immunotherapy Response Rajan Kulkarni, MD, PhD, Assistant Professor, Dermatology and CEDAR/Knight Cancer Institute, Oregon Health and Science University PD-1 inhibitors have promising efficacy in several cancers. Circulating tumor cell (CTC) PD-L1 levels could supplement tissue PD-L1 biopsy results by sampling from disseminated tumor sites. Towards establishing CTCs as a screening tool, we developed a protocol to isolate CTCs at high purity and immunostain for PD-L1. Monitoring of PD-L1 expression on CTCs could be an additional biomarker that may help in determining response to immunotherapies. 11:55 Molecular Heterogeneity as Expressed in Cells from Liquid Biopsies Using CellSearch and DepArray in Multiple Myeloma Steven Gross, PhD, Head, CellSearch Assay Development, Research & Development, Menarini Silicon Biosystems Inc Exploring the molecular heterogeneity as expressed in cells using CellSearch and DepArray technologies in multiple myeloma and other cancers. 12:10 pm Sponsored Presentation (Opportunity Available) 12:25 Session Break 12:35 Luncheon Presentation I : Esophageal Adenocarcinoma: Circulating Tumor Cells in Multimodal Treatment Protocols Jasmina Kuvendjiska, PhD, Surgery, Freiburg University Our study was designed as a pilot study of the ESOPEC-Trial. We evaluated the presence and morphology of CTCs during the treatment period. The experimental results will be correlated with patients’ overall and relapse-free survival. 1:05 Luncheon Presentation II to be Announced 1:35 Refreshment Break in the Exhibit Hall with Poster Viewing TECHNOLOGIES FOR LIQUID BIOPSY 2:05 Chairperson’s Remarks Steven A. Soper, PhD, Professor, Micro and Nanofabricated Tools for Biological Discovery and Medical Diagnostics, University of Kansas 2:10 Diagnosing Disease with Rare Circulating EVs: Finding Heterogeneous, Nanoscale Needles in a Nanoscale Haystack David A. Issadore, PhD, Assistant Professor, Bioengineering & Electrical & Systems Engineering, University of Pennsylvania Circulating EVs contain a wealth of proteomic and genetic information, presenting an enormous opportunity for liquid biopsy. While microfluidics have been used to successfully isolate cells from complex samples, scaling these approaches for EV isolation has been limited by the low throughput and susceptibility to clogging of nanofluidics. Moreover, the analysis of EV biomarkers is confounded by substantial heterogeneity between patients and within a disease itself. To address these challenges, we developed a multichannel nanofluidic system to analyze crude clinical samples. Using this platform, we isolated EVs, profile the RNA cargo inside of these EVs, and apply a machine learning algorithm to generate predictive panels that could provide useful diagnostics for applications in traumatic brain injury and pancreatic cancer using both murine models and clinical samples. 2:40 Single Cell Morphogenomic and Morphoproteomic Profiling of Liquid Biopsy in Prostate Cancer Paymaneh D. Malihi, PhD Candidate, USC Michelson Center for Convergent Bioscience Peter Kuhn, PhD, Dean’s Professor of Biological Sciences and Professor of Biological Sciences, Medicine, Biomedical Engineering, and Aerospace and Mechanical Engineering and Associate Director, The Bridge, University of Southern California Tumor heterogeneity is prevalent in both treatment-naïve localized and end-stage metastasized prostate cancer, and may contribute to the broad range of clinical presentation, treatment response, and disease progression. High Definition-Single Cell Analysis enables morphoproteomic and morphogenomic profiling of single cells from touch preparations of tissue cores as well as liquid samples. HD-SCA platform enables real-time molecular profiling of cells and has the potential to elucidate the origin and evolution of metastatic tumor cells. 3:10 High Precision Isolation and Analysis of Exosomes Daniel T. Chiu, PhD, A. Bruce Montgomery Professor of Chemistry and Bioengineering, University of Washington We have recently developed microfluidic and nanofluidic systems for the isolation and analysis of exosomes, offering detailed molecular information with single-exosome resolution. Here, we will describe our technical approach, device performance, and the new information we learned about exosomes as revealed by the new measurements. 3:40 Presentation to be Announced 3:55 Talk Title to be Announced Christoph Mauracher, PhD, Managing Director, STRATEC Consumables GmbH 4:10 St. Patrick’s Day Celebration in the Exhibit Hall with Poster Viewing 5:00 Breakout Discussions in the Exhibit Hall Parallel Analysis of Circulating Biomarkers in Immunotherapy Genevieve Boland, MD, PhD, Director, Melanoma Surgery Program, Massachusetts General Hospital; Director, Surgical Oncology Research Laboratories, Massachusetts General Hospital; Assistant Professor, Harvard Medical School; Associate Member, Broad Institute Clinical application of blood-based biomarkers in melanoma Unmet clinical needs in blood-based biomarkers Microvesicle applications in immunotherapy Clinical Utility and Impact of Liquid Biopsy Rajan Kulkarni, MD, PhD, Assistant Professor, Dermatology and CEDAR/Knight Cancer Institute, Oregon Health and Science University Necessary features of technologies/tests for clinical utility Tumor information that is of clinical relevance Necessity for standardization The Importance and Challenge in CTC Culture Professor Yong-Jie Lu, MBBS, MD, PhD, Professor, Centre for Molecular Oncology, Barts Cancer Institute, Queen Mary University of London Why is CTC culture important? What is the challenge? Does it worth to try it? Alternative ways to avoid it? How can we success with CTC culture? The researcher, technology development and the funding supporter/policy marker. Deploying Bioengineered 3D Tumor Models into Clinical Practice and the Pharmaceutical Pipeline Aleksander Skardal, PhD, Assistant Professor, Regenerative Medicine, Wake Forest Institute for Regenerative Medicine, Wake Forest School of Medicine Where is the clinical enterprise and/or pharma in terms of seeing value in these model systems? How do we convince clinicians and more traditional scientists that these new technologies are important and effective? 3D models are typically better representations of in vivo biology; Why the slow adoption? (besides decreased throughput) Complexity (more cell types or multiple organoids per model system) versus scale-up/high throughput Simple tumor organoids (aka spheroids) are amenable to medium-high throughput screening. What about multi-organ system (aka body-on-a-chip)? Where in the pharmaceutical pipeline do more advanced systems that are less amenable to high throughput fall? Post-preclinical/pre-human Phase I? Serve as go/no go points? Isolation and Analysis of CTCs Min Yu, MD, PhD, Assistant Professor, Stem Cell Biology and Regenerative Medicine Member, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California Downstream analysis of circulating tumor cells Technologies for CTC isolation Experimental mouse models for metastasis analysis Using CTCs as liquid biopsy 6:00 Close of Day Day 1 | Day 2 | Day 3 | Download Brochure WEDNESDAY, MARCH 13 7:30 am Registration Open and Morning Coffee 8:00 Plenary Keynote Session > 10:00 Refreshment Break and Poster Competition Winner Announced in the Exhibit Hall CIRCULATING BIOMARKERS INFORMING CLINICAL TRIALS 10:50 Chairperson’s Remarks Stefanie Jeffrey, MD, John and Marva Warnock Professor, Surgery; Chief, Surgical Oncology Research, Stanford University School of Medicine 11:00 Utilizing Extracellular Vesicles for Prediction and Monitoring of Immunotherapy Responses in Melanoma Genevieve Boland, MD, PhD, Director, Melanoma Surgery Program, Massachusetts General Hospital; Director, Surgical Oncology Research Laboratories, Massachusetts General Hospital; Assistant Professor, Harvard Medical School; Associate Member, Broad Institute Immune checkpoint inhibitors (ICI) show therapeutic benefit in melanoma. We identified pretreatment and early on-treatment biomarkers that incorporate tumor and host immune status. Our findings suggest that peripheral blood-derived exosomes may serve as a non-invasive biomarker to probe tumor and immune responses to ICI therapy, functioning as both a predictive marker of ICI responsiveness as well as a monitoring tool for tumor persistence and immune activation. 11:30 Molecular Assessment of Circulating Extracellular Vesicles Toward Liquid Biopsy Diagnosis of Gastrointestinal Stromal Tumor and Ewing Sarcoma Andrew K. Godwin, PhD, Chancellors Distinguished Chair in Biomedical Sciences and Endowed Professor and Director, Molecular Oncology, Professor, Department of Pathology and Laboratory Medicine, University of Kansas Medical Center, Deputy Director, University of Kansas Cancer Center There is growing interest in exploiting circulating extracellular vesicles (EVs), primarily small bioactive nanovesicles (60–120 nm), known as exosomes, as developing sources of biomarkers for diagnosis and therapeutic response. However, EVs are heterogeneous vesicles released by both healthy and tumors cells, with the vast majority of circulating vesicles being derived from normal host cells. To establish their utility as valuable tools for the diagnosis of cancer and therapeutic monitoring of disease progression and response to therapy, we used molecular approaches to profile the proteome of Gastrointestinal Stromal Tumor (GIST)- and Ewing Sarcoma (EWS)-derived EVs, as well as other types of cancer, which provided insights into the oncogenic cargo of these tumor-derived EVs. Using proteins enriched in these different sarcomas, we have developed microfluidic platforms and immunocapture approaches to target tumor-associated EVs and in turn measure exosomal proteins and/or RNAs specific to a cancer subtype to further enhance the specificity of these liquid-based biopsies. 12:00 pm Clinical Trials – Liquid Biopsy in Lung Cancer Lecia V. Sequist, MD, Hematology/Oncology, Massachusetts General Hospital We will review the wide variety of liquid biopsy assays that have been used in clinical trials for lung cancer patients, and how this is affecting practice in the real world. 12:30 Session Break 12:40 Luncheon Presentation (Sponsorship Opportunity Available) or Enjoy Lunch on Your Own 1:10 Refreshment Break in the Exhibit Hall and Last Chance for Poster Viewing NEW MODELS OF MECHANISMS OF CANCER DISSEMINATION 1:50 Chairperson’s Remarks Shannon L. Stott, PhD, Assistant Professor, Department of Medicine, Massachusetts General Hospital, Harvard Medical School; BioMEMS Resource Center, The MGH Cancer Center 2:00 Advanced in vitro Cancer Models: Metastasis-on-a-Chip and Patient-Derived Organoid Technologies Aleksander Skardal, PhD, Assistant Professor, Regenerative Medicine, Wake Forest Institute for Regenerative Medicine, Wake Forest School of Medicine Most in vitro models of human tumors are too simplistic and fail to accurately recapitulate the in vivo tumor microenvironment. This presentation will describe 1) development of a microfluidic platform supporting observable metastasis from a primary site organoid to downstream target organoids, and 2) the translation of organoid technology to support models derived from clinical tumor biospecimens for personalized ex vivo drug screening and therapeutic efficacy identification prior to treatment. 2:30 Patient-Derived Circulating Tumor Cells Inform Mechanisms of Metastasis Min Yu, MD, PhD, Assistant Professor, Stem Cell Biology and Regenerative Medicine Member, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California Circulating tumor cells (CTCs) are expected to contain metastasis-initiating cells that can shed light on the mechanisms of cancer metastasis. However, due to limited patient-derived material, the metastatic capability of CTCs has yet to be proved. Using our recently established patient-derived CTC lines, we found that different patient CTC lines demonstrated distinct metastatic tissue tropisms in immunodeficient mice and identified associated pathways to specific organs via RNA-seq and ATAC-seq analysis. 3:00 From Sample-to-Single Cells-to-Answer – An Integrated Microfluidics Approach for Identifying Cancer Cells Abraham “Abe” Lee, PhD, William J. Link Professor and Chair, Department of Biomedical Engineering (BME); Professor, Mechanical & Aerospace Engineering; Director, NSF I/UCRC CADMIM Research Lab: Biomolecular Microsystems and Nano Transducers (BioMiNT), University of California, Irvine Liquid biopsy is performed by a microfluidic acoustic microstreaming device to isolate and target circulating tumor cells (CTCs) in whole blood. A key bottleneck is to identify the critical subpopulation of cells, often at single cell resolution among billions of cells in circulation. An in vitro perfused vascularized 3D tissue platform can then determine which CTCs can be disseminated to distant tissues through the circulatory system. 3:30 Session Break EXPANSION OF CIRCULATING TUMOR CELLS: A CHALLENGE! 3:40 Chairperson’s Remarks Catherine Alix-Panabières, PhD, Director, Laboratory of Rare Human Circulating Cells (LCCRH), Pathology and Onco-Biology Department, University Medical Center of Montpellier 3:45 Expansion of Patient-Derived Circulating Tumor Cells from Liquid Biopsy Chwee Teck (C.T.) Lim, PhD, NUS Society (NUSS) Professor, Department of Biomedical Engineering; Director, Biomedical Institute for Global Health Research and Technology; Founding Principal Investigator, Mechanobiology Institute, National University of Singapore Personalized therapy in cancer requires monitoring of patients’ individual response to treatment. One approach is to assess drug efficacy on circulating tumor cells (CTCs) obtained from patients’ blood samples (aka liquid biopsy) following ex vivo expansion into CTC clusters. We developed a simple microfluidics-based culture platform that allows co-culture of immune cells with CTCs (obtained after red blood cell lysis of patients’ blood) to promote CTC cluster formation. 4:15 Holy Grail and Big Challenge – Culture of Circulating Tumor Cells Yong-Jie Lu, MBBS, MD, PhD, Professor, Centre for Molecular Oncology, Barts Cancer Institute, Queen Mary University of London Circulating tumor cells (CTCs) provide a minimally invasive approach to access living cancer cells. The culture of CTCs enables the investigation of their biological features, mechanisms of cancer metastasis and the opportunity for in vitro therapeutic sensitivity tests. Challenge to CTC culture includes their rarity, small proportion of viable cells and unclear favorable growth condition. I will give a brief summary of culture strategies and our own experience. 4:45 Growing Organoids from CTCs: The Importance of CTC Input Andrew Rhim, PhD, Associate Director for Translational Research Ahmed Center for Pancreatic Cancer Research Assistant Professor of Internal Medicine UT MD Anderson Cancer Center Liquid biopsies have potential to inform personalized treatments and decision making in patients with cancer. While still in its infancy, the field has exploded, featuring numerous modalities and source material, including circulating tumor cells (CTCs), circulating nucleic acids, exosomes, and others. CTCs, however, hold the greatest promise, as not only do they contain all the tumor-derived material that are in the circulation, but if they could be cultured and propagated ex vivo, the prospect of functional analysis and direct drug combination testing could direct true individualized cancer care. Moreover, since CTCs are in the circulation, serial assay of CTCs, during the throes of treatment, could allow for nimble decision-making to thwart resistance. The major hurdle in the application of CTCs in the clinic is the inability to establish CTC cultures. Our laboratory has hypothesized that the major determinant of culture success is the number of CTCs seeded. This hypothesis is driven in part by evidence suggesting that a sub population of tumor cells retain stem cell like characteristics, called cancer stem like cells. These cells are theorized to be required to initiate tumors and metastases since they possess the capacity for self-renewal. In current protocols, at most 60ml of blood is phlebotomized for CTC isolation. However, we estimate that this yields far too few cancer stem like cells to achieve success in culture. Here we will review the efforts we have undertaken to achieve robust and routine cultures of CTCs, mostly by focusing on dramatically increasing CTC input with less emphasis on epithelial cell purity. In so doing, our preliminary data suggest real promise in our approaches. 5:15 Close of Conference Program Stay Late for: MARCH 14-15 TS4: Introduction to Liquid Biopsy for Cancer - Detailed Agenda Day 1 | Day 2 | Day 3 | Download Brochure

|
Suggested by
Société Francaise d'Immunologie
November 29, 2018 4:12 AM
|
Introduction: Multiple myeloma (MM) is a malignancy of terminally differentiated plasma cells. The high heterogeneity of MM cells is one of the major cause of disease relapse. Detection of circulating MM cells (CMMC) from peripheral blood is a useful procedure to investigate tumor heterogeneity and provides a painless alternative to the classic bone marrow biopsy to monitor disease progression. Here we demonstrate that the synergy between CellSearch® (CS) and DEPArray™ (DA) technologies can be used to identify, isolate and characterize at the genetic level single and pure CMMCs .
Methods: 4.0 ml of peripheral blood samples were obtained from 3 patients with MM. Putative CMMCs were enriched with CS using anti-CD138 or anti-CD138/CD38 as positive selection marker and subsequently stained with CD38-PE, CD19/CD45-APC immunofluorescent probes. Cells detection and enumeration was performed based on the co-localization of nuclei DAPI staining and CD38-PE. Single CMMCs (CD38+/CD19- and CD45-/DAPI+) and White Blood Cells (WBCs: CD38-/CD19+ or CD45+/DAPI+) were then isolated using the DA NxT system. Single cells genomic DNA was amplified using Ampli 1™ Whole Genome Amplification (WGA) kit and Illumina®-compatible libraries were obtained using Ampli 1™ LowPass kit and a high-throughput, customized automated protocol using Hamilton STARLet Liquid handler. Highly-multiplexed, genome-wide single-cell Low-Pass Copy Number Alteration (LPCNA) analysis was performed using HiSeq 2500 Illumina® platform.
Results: CS and DA workflow* enabled the isolation of 215 single CMMC, selected for LPCNA analysis. 42 single WBCs were also included as normal controls. Copy-number profiles of single CMMCs showed relevant gains and losses of chromosomal segments, as result of a high-level genomic instability. Notably, intra-patient CMMCs revealed overall conserved CNA patterns with subclonal alterations, suggesting a certain level of branched tumor evolution. Conversely, a higher degree of heterogeneity in CMMCs CNA profiles was observed among different patients. Interestingly, CNAs detected in all patients are located in regions containing genes involved in cell cycle regulation (MAPK, NOTCH pathways) and cell signaling (IL6R), which might be involved in proliferative processes and immuno-surveillance escape.
Conclusion: The combination of CS and DA workflow* with a streamlined automated protocol allowed to obtain hundreds of genomic libraries from pure single CMMCs. The presented workflow constitutes a non-invasive, rapid and high-throughput approach for characterizing MM tumor heterogeneity and progression, suggesting a possible future implementation in clinical applications.
*For Research Use Only. Not for use in diagnostic procedures.
Disclosures Raspadori: Menarini Silicon Biosystems: Employment. Forcato: Menarini Silicon Biosystems: Employment. Edoardo: Menarini Silicon Biosystems: Employment. Papadopulos: Menarini Silicon Biosystems: Employment. Ferrarini: Menarini Silicon Biosystems: Employment. Del Monaco: Menarini Silicon Biosystems: Employment. Terracciano: Menarini Silicon Biosystems: Employment. Morano: Menarini Silicon Biosystems: Employment. Gross: Menarini Silicon Biosystems: Employment. Bolognesi: Menarini Silicon Biosystems: Employment. Buson: Menarini Silicon Biosystems: Employment. Fontana: Menarini Silicon Biosystems: Employment. Connelly: Menarini Silicon Biosystems, Inc.: Employment, Other: Chief R&D Officer, USA. Simonelli: Menarini Silicon Biosystems: Employment. Medoro: Menarini Silicon Biosystems: Employment. Manaresi: Menarini Silicon Biosystems: Employment.

|
Scooped by
Gilbert C FAURE
November 27, 2018 4:16 AM
|
Peter Kuhn, who developed a “liquid biopsy” to spot cancer cells in the circulatory system, came to USC to help anchor the USC Michelson Center for Convergent Bioscience. Convergence — the focus on wicked problems requiring the blending of multiple disciplines — is what attracted Kuhn to USC.

|
Scooped by
Gilbert C FAURE
November 23, 2018 1:43 PM
|
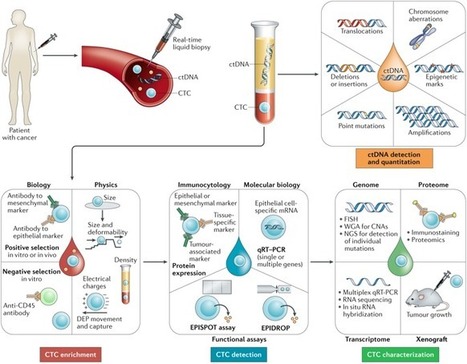
Abstract“Liquid biopsy” was introduced as a new diagnostic concept in 2010 for the analysis of circulating tumor cells (CTCs) and has been now extended to material (in particular DNA) released by tumor cells in the peripheral blood of cancer patients.

|
Scooped by
Gilbert C FAURE
September 5, 2018 12:04 PM
|
|

|
Scooped by
Gilbert C FAURE
November 25, 2019 12:11 PM
|
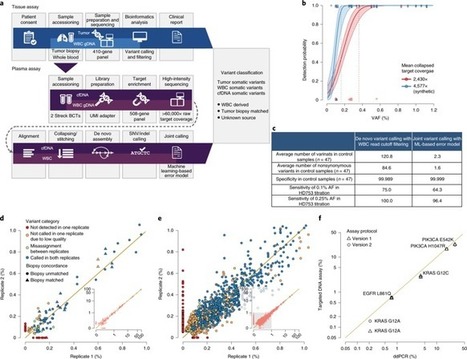
Accurate identification of tumor-derived somatic variants in plasma circulating cell-free DNA (cfDNA) requires understanding of the various biological compartments contributing to the cfDNA pool. We sought to define the technical feasibility of a high-intensity sequencing assay of cfDNA and matched white blood cell DNA covering a large genomic region (508 genes; 2 megabases; >60,000× raw depth) in a prospective study of 124 patients with metastatic cancer, with contemporaneous matched tumor tissue biopsies, and 47 controls without cancer. The assay displayed high sensitivity and specificity, allowing for de novo detection of tumor-derived mutations and inference of tumor mutational burden, microsatellite instability, mutational signatures and sources of somatic mutations identified in cfDNA. The vast majority of cfDNA mutations (81.6% in controls and 53.2% in patients with cancer) had features consistent with clonal hematopoiesis. This cfDNA sequencing approach revealed that clonal hematopoiesis constitutes a pervasive biological phenomenon, emphasizing the importance of matched cfDNA–white blood cell sequencing for accurate variant interpretation. Ultra-sensitive cell-free DNA (cfDNA) sequencing uncovers clonal hematopoiesis as a major source of somatic cfDNA variants in healthy individuals and patients with cancer, and underscores the importance of matched white blood cell DNA sequencing in liquid biopsy procedures.

|
Scooped by
Gilbert C FAURE
October 20, 2019 12:37 AM
|
The detection of circulating tumor cells (CTCs), an approach considered to be “liquid biopsy”, is crucial in cancer diagnosis, monitoring and prognosis. However, the extremely large number of blood cells challenges the rare CTC isolation and enrichment.

|
Scooped by
Gilbert C FAURE
August 13, 2019 2:29 PM
|
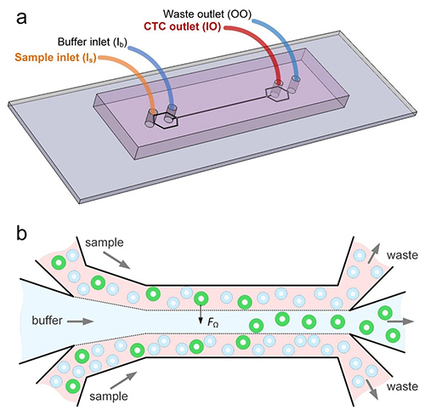
University researchers develop microfluidic device that partitions cancer cells according to size in effort to create a useful liquid biopsy method.

|
Scooped by
Gilbert C FAURE
July 26, 2019 8:35 AM
|
A circulating cancer cell (pink) attaches to carbon nanotube surface; white blood cells (blue) do not adhere and are later washed away.Using the tendency of circulating cancer cells to adhere to c…...

|
Scooped by
Gilbert C FAURE
June 17, 2019 2:21 AM
|
Clin Exp Med. 2019 Jun 12. doi: 10.1007/s10238-019-00563-w.[Epub ahead of print] Review...

|
Scooped by
Gilbert C FAURE
May 8, 2019 3:52 AM
|
Advancing technology is allowing scientists increasingly to search for tiny signs of cancer and other health issues in samples of patients' blood and urine. These "liquid biopsies" are less invasive than a traditional biopsy, and can provide information about what's happening...

|
Scooped by
Gilbert C FAURE
March 29, 2019 2:39 PM
|
March 2019—Cancer, liquid biopsy, pathology. The standard riff for talking about a promising new cancer test should be familiar to anyone within sneezing distance of a laboratory

|
Scooped by
Gilbert C FAURE
February 5, 2019 6:53 AM
|
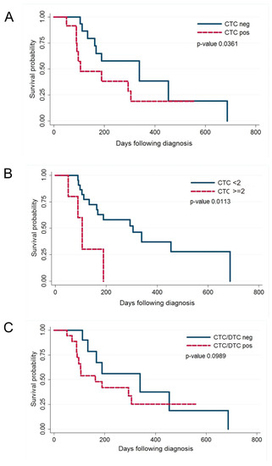
In human breast cancer, both circulating tumour cells (CTCs) in peripheral blood and disseminated tumour cells (DTCs) in the bone marrow are predictive of short survival and may be used as liquid biopsy to guide therapy.

|
Scooped by
Gilbert C FAURE
December 6, 2018 12:08 PM
|
This Review discusses the challenges in accurately identifying rare prostate circulating tumour cells or bone marrow-derived disseminated tumour cells and the resulting need for prostate-specific, sensitive biomarkers for the detection of these cells in liquid biopsy samples.

|
Scooped by
Gilbert C FAURE
November 28, 2018 1:39 PM
|
The initial results of an ongoing study show that a liquid biopsy has advantages over a tissue biopsy for people with lung cancer.

|
Scooped by
Gilbert C FAURE
November 24, 2018 2:10 PM
|
"Liquid biopsy" was introduced as a new diagnostic concept in 2010 for the analysis of circulating tumor cells (CTCs) and has been now extended to mat...

|
Scooped by
Gilbert C FAURE
September 13, 2018 3:43 AM
|
Abstract Background The aim of this study was to determine a correlation between benign and malignant lung solitary pulmonary nodules (SPN), and analyze the association between circulating tumor cell (CTC) levels and different subtypes of lung adenocarcinoma. Methods A total of 200 patients (80 with SPNs and 120 diagnosed with lung cancer) were included in the study. The CTC levels were quantified by identifying the folate receptor on the surface of tumor cells; clinical tumor specific markers were detected by biochemical immunization. The content of peripheral blood CTCs in benign and malignant lung SPN patients was detected and the differences in preoperative CTC levels in different pathological subtypes were analyzed. Based on the collected data, receiver operating characteristic curves were calculated and the rate of lung cancer was predicted. Results The peripheral blood CTC levels in patients with malignant lung SPNs were higher than in patients with benign SPNs. The maximum nodule diameter, carcinoembryonic antigen, and CTC levels were independent risk factors for malignant lung SPNs. The peripheral blood CTC levels in patients with stage III–IV lung adenocarcinoma were higher than in stage I–II patients. The peripheral blood CTC levels in patients with microinvasive and invasive adenocarcinoma were higher than in adenocarcinoma in situ patients. The CTC levels in the peripheral blood of patients with maximum tumor diameter > 2 cm were higher than in patients with tumors < 2 cm. Conclusion The detection of CTCs can be used as a biomarker for screening SPNs and diagnosing early‐stage lung cancer. Using the combination of CTC levels and CEA significantly improves the efficacy of lung adenocarcinoma diagnosis. Introduction Lung cancer is a significant malignancy; United States (US) cancer statistics estimate that 83 550 men and 70 500 women will die from lung cancer in 2018, ranking first of all malignancies.1 Although methods for early screening, diagnosis, and multidisciplinary treatment have been developed, the overall survival rate of lung cancer patients has not greatly improved and patients are usually diagnosed in late stages. Even if surgery is performed at an early stage of the disease, there is still a 25–50% recurrence rate.2 Solitary pulmonary nodules (SPNs) are single, well‐defined lesions of a diameter of ≤ 3 cm that are completely surrounded by gas‐containing lung tissue, without pulmonary atelectasis, hilar enlargement, or pleural effusion.3 At present, SPNs are a focus of research, primarily related to imaging, biomarkers, and histopathology. There are few biomarkers for lung cancer during early stage, thus it is difficult for clinicians to determine optimal treatment.4 With advances in precision medicine and progress in technology, the detection of circulating tumor cells (CTCs) in peripheral blood is a simple and noninvasive “liquid biopsy” method.5 CTCs can be detected earlier than imaging and clinical symptoms. They are collectively referred to tumor cells, metastasize in blood circulation, and may lead to tumor progression and spread throughout the body. In addition, they are often used as a predictor in lung cancer patients for progression‐free and overall survival.6 Real‐time dynamic analysis of CTCs may provide a new metric for the individualized treatment of cancer patients.7 More importantly, this method has become important to evaluate the status of tumors.8 Folate receptor (FR) is highly expressed in many epithelial‐derived malignant tumor cells, and is significantly upregulated in 75.7% of non‐small cell lung cancer (NSCLC) patients.9 There are approximately 500 000 receptors on the surface of each lung cancer cell, but no expression in normal human blood cells. Consequently, FR is an ideal detection point for CTCs in patients with lung cancer. FR‐positive CTCs can be detected by ligand‐targeted enzyme‐linked polymerization4 and have shown diagnostic promise for various cancers.10 For instance, FR expression is higher in both the serum and urine specimens of patients with bladder transitional cell carcinoma compared to a control group.11 FR‐positive CTCs are used as a novel diagnostic biomarker for lung cancer.12 Therefore, we hypothesized that the presence of FR‐positive CTCs were related to the pathological type, stage, and size of lung tumors. In this study, identification of FR in tumor cells was used to quantify CTC levels in benign and malignant SPNs and for the early detection and adjuvant diagnosis of lung cancer. Methods Case selection In this prospective clinical study, 200 patients (80 with SPNs and 120 with lung cancer) diagnosed at the Department of Respiratory Medicine, Thoracic Surgery, and Oncology of the First Affiliated Hospital of Soochow University between September 2016 and June 2017, were enrolled. All patients had a histopathological diagnosis report. Clinical tumor node metastasis (TNM) staging was confirmed based on 7th edition International Association for the Study of Lung Cancer guidelines (2009).13 All patients (aged 18–80 years) included had good compliance, had not received any anti‐tumor therapy, and did not have other types of malignant tumors, or abnormal liver and kidney function. Patients voluntarily participated in the study and signed informed consent. The ethics committee of the First Affiliated Hospital of Soochow University approved the study. Detection of folate receptor (FR)‐positive circulating tumor cells (CTCs) A total of 3 ml antecubital vein blood was extracted from each patient, anticoagulated by ethylene‐diamine‐tetraacetic acid, and stored at 4°C. Blood treatment was completed within 24 hours. CTCs were captured in peripheral blood by immunomagnetic bead negative enrichment and FR‐positive CTCs were quantitatively detected by ligand‐targeted PCR. GenoBiotech Co., Ltd (Shanghai, China) provided an FR‐positive CTC test kit. One FR‐positive cell detected in 3 mL of blood was defined as 1 folate unit (FU).12 Single blind protocols were followed in this study. According to kit instructions, red blood cell lysate (1:4) was added to 3 mL whole blood samples and lysed at 4°C for 15 minutes to remove red blood cells. Then, 150 μL anti‐CD45 magnetic beads and 50 anti‐CD14 magnetic beads were added and incubated for 30 minutes at 4°C to remove leukocytes and macrophages, respectively. The enriched CTCs were added to 10 μL of the probe labeling solution, which contained the tumor‐specific folate ligand‐oligonucleotide conjugate, and incubated at room temperature for 40 minutes. Washing buffer (1 mL) was then added into the samples, which were centrifuged at 4°C and 500 g for 10 minutes three times to remove unbound probes. Finally, 120 μL of elution buffer was added and incubated for 2 minutes at 4°C to elute the bound probe. The bound probe was collected by centrifugation and 24 μL neutralization buffer was added for amplification of fluorescence quantitative PCR. ABI7300 instruments (Life Technologies, Carlsbad, CA, USA) were used to collect PCR signals and data. The reaction conditions were: 95°C, 2 minutes; 40°C, 30 seconds; 72°C, 30 seconds; 8°C, 5 minutes; 40 cycles, 95°C, 10 seconds; 35°C, 30 seconds; and 72°C, 10 seconds.14 Detection of clinical tumor‐specific biomarkers Gastrin releasing peptide (pro‐GRP) was detected by chemiluminescent microparticle immunoassay (CMIA); pro‐GRP in human serum was quantitative determined on the ARCHITECTi system (Abbott Laboratories, Chicago, IL, USA). The normal reference range of pro‐GRP is 0–70 pg/mL. A Roche Cobas 601 automatic chemical analyzer (Roche, Basel, Switzerland) was used to detect the expression of serum tumor biomarkers, including neuron‐specific enolase (NSE), carcinoembryonic antigen (CEA), cytokeratin fragment 19 (CYFRA21‐1), and squamous cell carcinoma (SCC) antigen. The normal reference ranges of these biomarkers were: CEA < 5.0 μg/L, CYFRA21‐1 < 3.3 μg/L, NSE < 16.3 μg/L, SCC < 1.5 μg/L, and pro‐GRP < 70 pg/mL. Statistical analysis SPSS version 20.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. The continuous variables that corresponded to normal distribution are expressed as mean ± standard deviation, continuous variables that did not follow normal distribution are expressed as medians, and categorical variables are expressed as frequency (%). Comparison of the measurement data between the groups was performed using an independent t‐test. The non‐parametric rank sum test was used for data of non‐normal distribution. The Enter method of logistic regression analysis was used for independent analysis of influencing factors: A receiver operating characteristic (ROC) curve was plotted with sensitivity as the ordinate and specificity as the abscissa. The Youden index was calculated using the ROC curve. The highest cutoff point of the Youden index was determined to be the best critical point for a diagnosis of lung cancer by FR‐positive CTCs. Results CTC levels in patients with benign malignant lung nodules Of the 80 patients with SPNs included in the study, 50 were confirmed to have malignant SPNs (62.5%): 32 had adenocarcinoma, 8 SCC, 5 small cell lung cancer (SCLC), and 1 had a carcinoid. The remaining 30 (32%) patients had benign SPNs: 20 had hamartomas, 4 inflammatory pseudotumors, 3 sclerosing hemangiomas, and 3 had tuberculosis. Patient characteristics by SPN and CTC levels are listed in Tables 1 and 2, respectively. Variables Patients with benign SPN (n = 30) Patients with malignant SPN (n = 30) Age (years) ≤ 60 12 15 > 60 18 35 Gender Male 8 27 Female 22 23 Smoking history Yes 8 30 No 22 20 The largest diameter of nodules (cm) ≤ 0.8 20 14 0.9–3.0 10 36 CEA Normal 22 10 Abnormal 8 40 SCC Normal 16 21 Abnormal 14 29 Pro‐GRP Normal 18 24 Abnormal 12 26 CYFRA21‐1 Normal 14 14 Abnormal 16 36 NSE Normal 13 18 Abnormal 17 32 Number of patients with CTCs Normal 23 15 Abnormal 7 35 CEA, carcinoembryonic antigen; CTC, circulating tumor cell; CYFRA, cytokeratin fragment 19; GRP, gastrin releasing peptide; NSE, neuron‐specific enolase; SCC, squamous cell carcinoma; SPN, solitary pulmonary nodule. Clinical pathological factors CTC level, median (quartile) P Age (year) 0.828 ≤ 60 8.10 (7.43–9.67) > 60 8.35 (7.80–9.57) Gender 0.903 Male 9.05 (8.49–10.52) Female 8.10 (7.21–9.01) Smoking history 0.861 Yes 10.10 (8.74–10.49) No 8.10 (6.804–8.755) Largest diameter of nodules (cm) 0.341 ≤ 0.8 8.30 (7.39–9.38) 0.9–3.0 8.47 (7.88–9.79) CTC, circulating tumor cell. To evaluate the diagnostic efficacy of FR‐positive CTCs for the early diagnosis of lung cancer, the data of malignant SPN patients were analyzed using the Mann–Whitney U test. As shown in Figure 1, the median CTC level in malignant SPN patients was 9.79 (range: 8.98–10.60) units, which was significantly higher than 6.66 (range: 5.81–7.51) in patients with benign SPN disease. The difference was statistically significant (P < 0.001). Multivariate logistic regression analysis was performed to determine what factors were predictive of cancer. Benign and malignant SPNs were used as the dependent variable and each demographic factor was used as an independent variable. The maximum diameter of nodules, CEA, and CTC levels were independent risk factors for malignant SPN disease (P < 0.05) (Table 3). Variables β SE Wald χ2 P OR 95% CI CTCs 1.969 0.760 6.709 0.010 7.162 1.615–31.772 CEA 1.724 0.708 5.929 0.015 5.606 1.400–22.450 SCC 0.704 0.783 0.810 0.368 2.022 0.436–9.380 NSE 0.392 0.680 0.333 0.564 1.480 0.391–5.607 CYFRA21‐1 1.288 0.872 2.182 0.140 3.624 0.657–2.100 Pro‐GRP 0.280 0.808 0.120 0.729 0.755 0.155–3.682 Largest nodule diameter 1.590 0.716 4.931 0.026 4.903 1.205–19.948 CI, confidence interval; CEA, carcinoembryonic antigen; CTC, circulating tumor cell; CYFRA, cytokeratin fragment 19; GRP, gastrin releasing peptide; NSE, neuron‐specific enolase; OR, odds ratio; SCC, squamous cell carcinoma; SE, standard error. Differences in peripheral blood CTC levels in lung cancer patients Of the 120 patients with lung adenocarcinoma included in the study, 40 had adenocarcinoma in situ (AIS), 41 had microinvasive adenocarcinoma (MIA), 23 had invasive adenocarcinoma (IA), and 16 had IA mutations. Based on TNM staging, 41 patients were at stage I, 36 at stage II, 33 at stage III, and 10 patients were at stage IV. Furthermore, 58 patients had highly differentiated tumors, 25 had moderately differentiated tumors, and 17 had poorly differentiated tumors. The other factors are shown in Table 4. Clinical pathological factors Cases (n = 120) Average age (years) 59.4 (25–85) Gender: (male/female) 49/71 Smoking history 61 (50.8%) Largest diameter of nodules (cm) 4.2 cm (0.7–12) T staging T1 63 (52.5%) T2 18 (15.0%) T3 23 (19.2%) T4 10 (8.3%) N staging N0 41(34.2%) N1 37 (30.8%) N2 36 (30.0%) N3 6 (5%) M staging M0 110 (91.7%) M1 10 (8.3%) Histological grade G1 41 (34.2%) G2 46 (38.3%) G3 33 (27.5%) TNM staging I 41 (34.2%) II 36 (30.0%) III 33 (27.5%) IV 10 (8.3%) Pathological subtype Adenocarcinoma in situ 40 (33.3%) Microinvasive adenocarcinoma 41 (34.2%) Invasive adenocarcinoma 23(19.2%) Invasive adenocarcinoma variants 16(13.3%) TNM, tumor node metastasis. As shown in Figure 2 and Table 5, the median CTC level in the lung adenocarcinoma group was 10.65 units (range: 9.93–11.37), and 6.66 (5.81–7.51) in the benign SPN group. The results of the Mann–Whitney U test showed that CTC levels in the lung adenocarcinoma group were higher than in the benign lung SPN group, and the difference was statistically significant (P < 0.001). In patients with lung adenocarcinoma, CTC levels in the peripheral blood of primary tumors with a maximum diameter > 2 cm were higher than those with a maximum diameter of ≤ 2 (P < 0.001). CTC levels in patients at stage III–IV lung adenocarcinoma were significantly higher than patients at stage I–II (P < 0.001). The levels of peripheral blood CTCs in patients with MIA and IA were significantly higher than in patients with AIS (MIA and IA together versus AIS: P < 0.03, IA: P < 0.002). Clinicopathological factors CTC level, median (quartile) P Average age (years) 0.662 ≤ 60 10.42 (8.98–11.87) > 60 10.24 (9.94–11.59) Gender 0.872 Male 10.72 (9.56–11.89) Female 10.60 (9.67–11.54) Smoking history 0.366 Yes 10.97 (9.89–12.05) No 10.31 (9.35–11.28) The largest diameter of nodules (cm) ≤ 2 cm 9.22 (8.06–10.38) 0.001 > 2 m 11.56 (10.69–12.43) Histological grade G1 10.27 (9.33–11.21) 0.593 G2 10.59 (9.31–11.87) G3 11.21 (9.58–12.84) TNM staging I and II staging 9.08 (8.47–9.69) 0.000 III and IV staging 13.49 (12.13–14.84) Pathological subtype Adenocarcinoma in situ 9.18 (8.29–10.06) 0.015 Microinvasive adenocarcinoma 11.07 (9.97–14.45) Invasive adenocarcinoma 12.38 (10.32–14.45) Invasive adenocarcinoma variants 10.77 (7.99–13.56) CTC, circulating tumor cell; TNM, tumor node metastasis. Receiver operating characteristic curve analysis To further analyze the potential value of FR‐positive CTC detection methods to diagnose lung cancer, all patients with lung cancer were included as a case group and patients with benign SPN as a control to create an ROC curve. Using sensitivity as the ordinate and 1‐specificity as the abscissa, ROC analysis demonstrated an area under the curve (AUC) of 0.836 and a 95% confidence interval (CI) 0.770–0.902). The Youden index was utilized to select the best cutoff value. As demonstrated in Figure 3, when sensitivity + specificity‐1 reached a maximum, the CTC value was 8.35, and a CTC level > 8.35 units was positive. Taking 8.35 units as the cutoff value for diagnosis, the diagnostic sensitivity of lung cancer patients was 70.2%, with a specificity of 79.3%. To further analyze the efficacy of FR‐positive CTC detection in patients with different pathological types of lung adenocarcinoma, the sensitivity in patients with AIS, MIA, invasive glands, and IA variants were 60%, 73.2%, 73.9%, and 75%, respectively. Effect of combining logistic regression multivariate analysis of CTCs and clinical biomarkers for the detection of lung adenocarcinoma Benign lung SPN and lung adenocarcinoma were used as dependent variables, while CTC levels and clinical biomarkers were used as independent variables for multivariate logistic regression analysis. As shown in Table 6, CTCs and CEA were independent risk factors for lung adenocarcinoma (P < 0.021 and P < 0.001, respectively). Variables β SE Wald χ2 P OR 95% CI CTCs 1.256 0.543 5.361 0.021 3.512 1.213–10.169 CEA 1.710 0.517 10.95 0.001 5.528 2.008–15.221 SCC 0.064 0.507 0.016 0.900 1.066 0.394–2.881 NSE 0.234 0.439 0.283 0.595 10 263 0.534–2.990 CYFRA21‐1 0.483 0.493 0.959 0.327 1.621 0.616–4.263 Pro‐GRP 0.304 0.491 0.384 0.535 1.356 0.518–3.550 Tumor size 1.005 0.533 3.919 0.058 2.873 1.011–8.167 CI, confidence interval; CEA, carcinoembryonic antigen; CTCs, circulating tumor cells; CYFRA, cytokeratin fragment 19; GRP, gastrin releasing peptide; NSE, neuron‐specific enolase; OR, odds ratio; SCC, squamous cell carcinoma; SE, standard error. Discussion In 1869, an Australian physician, Thomas Ashworth, dissected a patient with advanced stage cancer and accidentally discovered cells in his peripheral blood that resembled the size and shape of the primary tumor, and subsequently proposed the concept of circulating tumor cells (CTC).15 Based on Ashworth's discovery, British pathologist, Stephen Paget proposed the well‐known hypothesis of seed and soil in 1889, successfully explaining the mechanism of tumor recurrence and metastasis.16 Choosing the right tumor‐specific antigen target is key to the success of CTC detection technology. Today, conventional hematology and high‐resolution imaging tests are still inadequate to allow early diagnosis, evaluation of efficacy, assessment of prognosis, and individualized treatment of lung cancer patients. Fortunately, CTC detection, which measures metastasis and recurrence, could make up for the deficiencies of these other detection methods. The results of this study were consistent with our speculation, that peripheral blood CTCs levels in patients with stage III–IV lung adenocarcinoma were higher than in stage I–II patients. The peripheral blood CTC levels in patients with MIA and IA were higher than those in patients with AIS. The CTC levels in the peripheral blood of patients with maximum tumor diameter (MTD) > 2 cm were higher than in patients with MTD < 2 cm. Carcinoembryonic antigen is one of the most widely used tumor biomarkers and the most valuable indicator to diagnose lung adenocarcinoma. CYFRA21‐1, a soluble fragment of cytokeratin 19 is mainly suitable for the early diagnosis of NSCLC, observation of efficacy, and evaluation of prognosis, especially for SCC with relatively high sensitivity and specificity.17 Pro‐GRP is a neuropeptide hormone, one of the molecules associated with neuroendocrine‐derived tissues and tumors. It is usually secreted by SCLC cells, acting as a tumor biomarker of SCLC, and contributes to the differential diagnosis of SCLC and NSCLC.18 Moreover, NSE is the key enzyme for adenosine triphosphate synthesis in glycolysis and is considered the most sensitive and specific biomarker for SCLC.19 The FR has a strong specificity for the tissue of a tumor and is highly expressed in lung cancer cells, particularly on the surface of NSCLC cells. The FR level in each cell surface is up to hundreds of thousands, while almost no FR expression is found in healthy human blood cells.20 Therefore, FR it is an ideal screening target for CTCs. A combination of FR‐positive CTC detection kits, enriched immunomagnetic bead negative screening, and targeted PCR technology could be used for the auxiliary diagnosis of various types and stages of lung cancer. In this study, the sensitivity of FR‐positive CTCs in the peripheral blood of patients with benign and malignant SPNs was evaluated. The average CTC level in malignant lung SPN patients was 9.79 (8.98–10.60) units, which was significantly higher than the average of 6.66 (5.81–7.51) in benign SPN patients. In addition, multivariate regression analysis showed that CTC level, CEA, and maximum nodule diameter were independent risk factors for malignant lung cancer patients, suggesting that detecting CTC levels combined with CEA could improve diagnostic efficacy for malignant lung SPNs. Analysis of these results revealed that the detection methods of FR‐positive CTCs can be used to screen biomarkers to isolate pulmonary nodules and diagnose early lung cancer. In a previous study, CTCs and serum/plasma markers were used as prognostic and predictive biomarkers for early‐stage NSCLC.21 Interestingly, detecting CEA/CK/CD133 messenger RNA in tumor drainage blood is reported to have prognostic value in CRC patients with Dukes’ stage B and C.22 Furthermore, breast cancer could be classified and individualized by detecting the level and characteristics of CTCs, which might be helpful to find the most timely and optimized regimens.23 More importantly, the role of CTCs in lung tumor progression and metastasis depends both on obtaining sufficient numbers and the enumeration of CTCs for downstream assays.24 The potential role of CTCs for the early diagnosis of tumors, to assess prognosis, evaluate efficacy, develop targeted drugs, and individualize treatment is an issue of great importance.25 The results of previous studies have confirmed that the greater the number of CTCs detected in peripheral blood, the greater the risk of adverse prognosis in lung cancer patients.26 In this study, the value of FR‐positive CTC assays was used to diagnose lung adenocarcinoma. Consistent with previous studies, our ROC curve and Youden index indicated a useful cutoff value of 8.35 units.27 The diagnostic sensitivity and specificity of our sample of lung adenocarcinoma patients were 70.2% and 79.3%, respectively. To further analyze the efficacy of FR‐positive CTC detection in patients with different pathological types of lung adenocarcinoma, the sensitivity in patients with AIS, MIA, and invasive glands, and IA variants were 60%, 73.2%, 73.9%, and 75%, respectively. All results had satisfactory diagnostic validity (P < 0.05). The MTD was used as an indicator for tumor burden. Patients with lung cancer were divided into MTD ≤ 2 cm and >2 cm groups by MTD mean. Lung cancer patients with a heavy tumor burden had higher CTC levels. Measuring the CTC level and CEA could be a method to improve diagnostic efficacy for lung adenocarcinoma. In conclusion, detection of FR‐positive CTCs can be used as a potential molecular marker for adjuvant diagnosis and early detection of lung cancer in patients with lung adenocarcinoma. However, this study also has some limitations. As a single‐center sample study, there may be certain bias in the selected threshold of the subjects, thus a larger sample is required for further research. Acknowledgments The authors would like to thank all of the patients for their participation and cooperation. This work was supported in part by grants from the National Natural Science Foundation of China (8167110475) and Suzhou City's Science and Technology Plan Project (SYS201529). Disclosure No authors report any conflict of interest. References
|

 Your new post is loading...
Your new post is loading...
 Your new post is loading...
Your new post is loading...