 Your new post is loading...
 Your new post is loading...

|
Scooped by
Gilbert C FAURE
June 11, 2024 3:19 AM
|
Urticaria is an inflammatory skin disorder that affects up to 20% of the world population at some point during their life. It presents with wheals, angioedema or both due to activation and degranulation of skin mast cells and the release of histamine and other mediators. Most cases of urticaria are acute urticaria, which lasts ≤6 weeks and can be associated with infections or intake of drugs or foods. Chronic urticaria (CU) is either spontaneous or inducible, lasts >6 weeks and persists for >1 year in most patients. CU greatly affects patient quality of life, and is linked to psychiatric comorbidities and high healthcare costs. In contrast to chronic spontaneous urticaria (CSU), chronic inducible urticaria (CIndU) has definite and subtype-specific triggers that induce signs and symptoms. The pathogenesis of CSU consists of several interlinked events involving autoantibodies, complement and coagulation. The diagnosis of urticaria is clinical, but several tests can be performed to exclude differential diagnoses and identify underlying causes in CSU or triggers in CIndU. Current urticaria treatment aims at complete response, with a stepwise approach using second-generation H1 antihistamines, omalizumab and cyclosporine. Novel treatment approaches centre on targeting mediators, signalling pathways and receptors of mast cells and other immune cells. Further research should focus on defining disease endotypes and their biomarkers, identifying new treatment targets and developing improved therapies. Urticaria is an inflammatory skin disorder that presents with itchy wheals and/or angioedema mediated by skin mast cells and release of histamine and other mediators. This Primer by Kolkhir et al. summarizes the epidemiology, mechanisms, diagnosis and treatment of urticaria, and discusses patient quality of life and open research questions for this condition.

|
Scooped by
Gilbert C FAURE
January 7, 2022 3:30 AM
|
Abstract This update and revision of the international guideline for urticaria was developed following the methods recommended by Cochrane and the Grading of Recommendations Assessment, Development...

|
Scooped by
Gilbert C FAURE
January 20, 2021 4:38 AM
|
Home: Reviews & News: Medical Journal Reviews: 2021: January 2021 Medical Journal Review January 2021 WAO Reviews – Editors' Choice Articles are selected for their importance to clinicians who care for patients with asthma and allergic/immunologic diseases by Juan Carlos Ivancevich, MD, and John J. Oppenheimer, MD - FACAAI - FAAAAI, WAO Reviews Editor. Asthma and COVID-19: Do we finally have answers? Eger K, Bel EH European Respiratory Journal 2020; in press https:/doi.org/10.1183/13993003.04451-2020 In this paper, Eger and Bel explore the impact of asthma on COVID 19. While it is well known that older age, obesity, cardiovascular disease, and diabetes are risk factors of poor COVID-19 outcome, much controversy surrounds asthma’s impact. The focus of this manuscript was 2 papers published in the same edition of the ERJ (Choi et al and Izquierdo et al). In summary, these large-scale studies confirmed previous findings regarding risk for asthma patients to develop (severe) COVID-19 – specifically that asthmatics appear to be slightly more susceptible to contracting COVID-19, but severe disease progression does not seem to be related to medication use, including asthma biologics, but rather linked to older age and co-morbidities. The authors stress the fact that often when examining studies of COVID-19 and asthma, potential bias factors have not been considered, leaving many questions unanswered. Furthermore, large-scale, multinational real-life studies with detailed information on asthma phenotype and medication usage in patients with a confirmed diagnosis of COVID-19 would be ideal to aid us in resolving these questions. The Metabolomics of Childhood Atopic Diseases: A Comprehensive Pathway-Specific Review Schjødt MS, Gürdeniz G, Chawes B Metabolites 2020;10(12):511 https://doi.org/10.3390/metabo10120511 Asthma, allergic rhinitis, food allergy, and atopic dermatitis are common childhood diseases with several different underlying mechanisms, i.e., endotypes of disease. In this review, the authors stress that metabolomics has the potential to identify disease endotypes, which could beneficially promote personalized prevention and treatment. They do a wonderful job of reviewing the metabolomics literature in children with atopic diseases, focusing on tyrosine and tryptophan metabolism, lipids (particularly, sphingolipids), polyunsaturated fatty acids, microbially derived metabolites (particularly, short-chain fatty acids), and bile acids. Specifically, tyrosine, 3-hydroxyphenylacetic acid, N-acetyltyrosine, tryptophan, indolelactic acid, 5-hydroxyindoleacetic acid, p-Cresol sulfate, taurocholic acid, taurochenodeoxycholic acid, glycohyocholic acid, glycocholic acid, and docosapentaenoate n-6 were identified in at least two studies as being impactful. They stress that altered metabolic pathways highlight some of the underlying biochemical mechanisms leading to these common childhood disorders, which in the future could provide utility in clinical practice. Much further work on this topic is still needed. Helicobacter pylori and skin disorders: a comprehensive review of the available literature Guarneri C, Ceccarelli M, Rinaldi L, Cacopardo B, Nunnari G, Guarneri F European Review for Medical and Pharmacological Sciences 2020;24(23):12267-12287 https://www.doi.org/10.26355/eurrev_202012_24019 Helicobacter pylori is a Gram-negative bacterium identified for the first time about 30 years ago and commonly considered as the main pathogenic factor of gastritis and peptic ulcer. Since then, it was found to be associated with several gastrointestinal and extra-gastrointestinal diseases, including skin disorders such as chronic urticaria, rosacea, lichen planus, atopic dermatitis, psoriasis, pemphigus vulgaris, vitiligo, primary cutaneous MALT-type lymphoma, sublamina densa-type linear IgA bullous dermatosis, primary cutaneous marginal zone B-cell lymphomas, and cutaneous T-cell pseudolymphoma. The aim of this review is to summarize the available studies regarding the topic and draw possible conclusions. The authors found that, overall, further clinical and laboratory studies are needed to assess the real plausibility and relevance of these associations, as well as the possible role of Helicobacter pylori with the underlying pathogenic mechanisms. Allergy prevention: An overview of current evidence Royal C, Gray C Yale Journal of Biology and Medicine 2020;93(5):689-698 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7757062/ There has been a rapid rise in allergic disorders worldwide, which has resulted in increased research into the determinants of allergy development in attempt to identify factors that may be manipulated to mitigate risk. Present literature demonstrates that an opportune window in immunological development appears to exist in early life, whereby certain exposures may promote or prevent the development of an allergic disposition. Furthermore, factors that affect the composition and diversity of the microbiome in early life may also be impactful. In this review, the authors explore the current literature and recommendations relating to exposures that may prevent allergy development or promote tolerance. They note several risk factors, including delivery by caesarean section, omission of breastfeeding, vitamin D insufficiency, and environmental exposures, such as cigarette smoke exposure, all increase the risk of an allergic predisposition. Likewise, they note several protective factors, including dietary diversity during pregnancy, lactation, and in infancy is protective. They also note that recommendations for food-allergen exposure have shifted from delayed introduction to early introduction as a tolerance-inducing strategy. Supplements such as probiotics and vitamins during pregnancy and infancy have yet to produce conclusive results for allergy prevention. Finally, they note that emollient use in infancy has not been shown to be protective against eczema or food allergy. The airways microbiome of individuals with asthma treated with high and low doses of inhaled corticosteroids Martin MJ, Zain NMM, Hearson G, Rivett DW, Koller G et al PLoS One 2020;15(12):e0244681 https://www.doi.org/10.1371/journal.pone.0244681 Inhaled corticosteroids (ICS) are the mainstay of asthma treatment, but evidence suggests a link between ICS usage and increased rates of respiratory infections. In this study, Martin and colleagues assessed the composition of the asthmatic airways microbiome in patients taking low and high dose ICS and the stability of the microbiome over a 2-week period. Sputum from each subject underwent DNA extraction, amplification and 16S rRNA gene sequencing of the bacterial component of the microbiome. Nineteen subjects returned for further sputum induction after 24 h and 2 weeks. A total of 5,615,037 sequencing reads revealed 167 bacterial taxa in the asthmatic airway samples, with the most abundant being Streptococcus spp. No significant differences in sputum bacterial load or overall community composition were seen between the low- and high-dose ICS groups; however, Streptococcus spp. showed significantly higher relative abundance in subjects taking low-dose ICS (p = 0.002). Furthermore, Haemophilus parainfluenzae was significantly more abundant in subjects on high-dose fluticasone propionate compared to those on high-dose budesonide (p = 0.047). There were no statistically significant changes in microbiota composition over a 2-week period. The authors note that the clinical implications for patients are not known, but suggest that this does provide a possible explanation for the increased risk of pulmonary infection seen in asthma and COPD, particularly with FP. More research is needed.

|
Scooped by
Gilbert C FAURE
July 4, 2020 2:35 AM
|
Abstract Background Chronic spontaneous urticaria (CSU) is considered an autoimmune disorder in 50% of cases at least, in which T‐ and mast cell mediators are considered to be the primary cause of ...
Monoclonal Antibody Therapies for Atopic Dermatitis: Where Are We Now in the Spectrum of Disease Management? JCAD Online Editor | February 1, 2019 This ongoing column explores emerging treatment options, drug development trends, and pathophysiologic concepts in the field of dermatology. J Clin Aesthet Dermatol. 2019;12(2):39–43 by James Q. Del Rosso, DO Dr. Del Rosso is Research Director of JDR Dermatology Research in Las Vegas, Nevada; is with Thomas Dermatology in Las Vegas, Nevada; and is Adjunct Clinical Professor (Dermatology) with Touro University Nevada in Henderson, Nevada. FUNDING: There was no funding related to the development, writing, or publication of this article. DISCLOSURES: Dr. Del Rosso is a consultant, speaker, and/or researcher for several companies who market products used in the management of atopic dermatitis or have compounds under development. These include Almirall, Dermira, Galderma, Genentech, LaRoche Posay, Leo Pharma, Loreal, Ortho Dermatologics, Pfizer, Promius, Regeneron, Sanofi-Genzyme, Skinfix, Sonoma, Sun Pharma, and Taro. Abstract: Atopic dermatitis (AD) is a chronic disorder that requires thorough patient education and a therapeutic management strategy designed to control flares, decrease recurrences, and reduce pruritus. In cases that cannot be controlled by proper skin care and barrier repair, topical therapy, and avoidance of triggers, systemic therapy is often required to control flares and maintain remission. It is important for clinicians to avoid becoming overly dependent on the intermittent use of systemic corticosteroid therapy to control flares, without incorporating other treatment options that might more optimally control AD over time. This article provides an overview of systemic therapies, including conventional oral therapy options and injectable biologic agents, that modulate the immune dysregulation in AD. Major emphasis is placed on the monoclonal antibodies currently available (e.g., dupilumab) for the treatment of AD, as well as those in latter stages of development, with a focus on agents targeting IL-4 and/or IL-13. KEYWORDS: Atopic dermatitis, calcineurin inhibitors, phosphodiesterase-4 inhibitors, immunosuppressants, interleukin-4, interleukin-13 Many patients with atopic dermatitis (AD) are able to control their disease primarily with topical agents, including corticosteroids, calcineurin inhibitors, phosphodiesterase-4 (PDE4) inhibitors, moisturizers/barrier repair agents, wet wraps, and the avoidance of triggers.1,2 However, it is important to better define the word “control,” as AD is a chronic disorder characterized by marked flares of eczema and pruritus, variable periods of persistent eczema of lesser severity with itching, and complete remission, all of which vary in intensity, frequency, and duration among each individual affected by AD. Marked flares can often be mitigated with topical agents of adequate potency and duration, and, in selected cases, in conjunction with short courses of systemic corticosteroid (CS) therapy. The most difficult therapeutic challenges in AD are effective control of eczematous dermatitis (eczema) and pruritus, both of which are persistent but of a lesser overall severity, and the maintenance of remission after control of disease flares.1–5 Many patients with AD, including the parents/guardians of children with AD, deserve a discussion of what options exist beyond topical management alone and intermittent systemic CS therapy. This discussion often needs to be initiated by the clinician, as patients with AD or other chronic disorders depend on their clinician to direct them toward what is likely to be the most effective treatment for them at any given point in time. There are only so many oral CS courses or intramuscular CS injections a clinician can prescribe to help control AD flares without tipping the benefit versus risk balance toward too much risk. This same principle also applies to repeated use of topical CS therapy, which can progress to use so frequent that the risk for adverse effects is increased significantly. Skin barrier repair agents and steroid-sparing topical agents (e.g., pimecrolimus, tacrolimus, crisaborole) provide marked benefit in some cases of AD, especially on certain anatomic sites or when the affected body surface area (BSA) is not too extensive.1–3 However, most patients with AD would benefit from systemic therapies that are designed to achieve optimal suppression of AD, including eczematous dermatitis and/or pruritus. Daily diffuse application of a well-formulated moisturizer for skin barrier maintenance and the application of prescription topical therapies to persistent AD lesions remain part of the standard therapeutic regimen, especially for localized refractory and lichenified sites.1–6 Finding the optimal balance of therapeutic choices varies among individual patients and requires careful consideration of the overall clinical situation and specific patient-related factors, such as age, severity of AD signs and symptoms, and patient and clinician comfort levels with the treatments selected. Ultimately, the clinician should identify what is most likely to achieve an optimal level of control and express their treatment recommendations to the patient with realistic confidence and a proper benefit versus risk discussion. The time has come for clinicians treating AD to consider moving from a rescue approach for flares to treating AD as a chronic, inflammatory, cutaneous and systemic disorder by using therapies that more selectively suppress the underlying disease pathophysiology, effectively treat eczema and pruritus, mitigate flares, and sustain long-term control of the disease. While topical therapies to manage epidermal barrier dysfunction and inflammation of AD should remain an important component of the total management approach for patients with AD, clinicians would be prudent to also consider therapies with better short-term and long-term safety profiles than the conventional oral agents that are currently available. In this article, an overview of the current conventional oral systemic therapeutic options for atopic dermatitis are presented, followed by an overview of the new systemic therapeutic options for AD, namely monoclonal antibody agents, including the currently available agent, duplimab, and other agents in latter stages of development, with a focus on compounds targeting IL-4 and/or IL-13. Other monoclonal antibodies that have been studied and/or are currently under evaluation for treatment of AD, such mepolizumab (anti-IL-5), nemolizumab (anti-IL-31), and omalizumab (anti-IgE), as well as other drug classes, will be discussed in future installments of “What’s New in the Medicine Chest.” Conventional Systemic Therapeutic Options for Atopic Dermatitis—Oral Agents When patients with moderate-to-severe AD and their clinicians are considering systemic therapy for AD, a variety of treatment options are available.3,5–12 Prior to 2018, available systemic therapies for AD were primarily oral agents, such as cyclosporin, methotrexate, azathioprine, and mycophenolate mofetil, all of which appear to modulate the underlying pathophysiologic pathways that contribute to AD.3,6–8 Each of these agents has variable amounts of data available regarding its use in children and adults for treatment of AD.3,6–12 However, none of these oral agents are approved by the United States Food and Drug Administration (FDA) for the treatment of AD, and all exhibit immunosuppressant properties.3,8 Oral antihistamines have also been used as part of the treatment regimen for AD, primarily as an adjunctive therapy to help reduce pruritus and/or decrease interference with sleep (i.e., sedating antihistamines).12 It is important to note that chronic or frequent use of systemic CS is best avoided in children and adults due to the risk of several significant AEs.6,10–12 Cyclosporin. Among the conventional systemic oral agents used in the management of AD, cyclosporin appears to exhibit the fastest onset of efficacy, but its use is limited by its safety profile, which includes risks of nausea, cephalgia, hypertension, nephrotoxicity, sequelae of chronic immunosuppression, gingival hyperplasia, and drug interactions.6,8,10 Cyclosporin is primarily recommended for treatment-resistant and/or uncontrolled AD, after which patients are usually transitioned to a safer, long-term approach; continuous use of cyclosporin beyond 12 to 24 months generally is not advisable.6,8,10 Methotrexate. Methotrexate therapy, another conventional systemic oral treatment for AD, can exhibit efficacy in as little as 4 to 8 weeks, but, like cyclosporin, warrants careful monitoring due to potential adverse events (AEs); these include nausea, bone marrow suppression (including pancytopenia), hepatotoxicity, pulmonary fibrosis, potential sequelae of immunosuppression, drug interactions, and the need to avoid alcohol intake.6,8,10 As with cyclosporin, long-term use of methotrexate should likely be avoided. Azathioprine. Azathioprine is another conventional systemic oral treatment option for AD, but it is not usually considered an initial systemic option due to its slower onset of efficacy and potential toxicities. Potential AEs include bone marrow suppression, increased malignancy risk, other sequelae of immunosuppression, severe nausea/vomiting, abdominal pain, hepatotoxicity, drug hypersensitivity syndrome, and risk for drug-drug interactions (e.g., allopurinol).6,8,10 Mycophenolate mofetil. Finally, although data for use of mycophenolate mofetil as a treatment option for AD are more limited than cyclosporin data, mycophenolate mofetil appears to be the safest oral agent, when compared with cyclosporin, methotrexate, and azathioprine; it has an efficacy onset range of 4 to 12 weeks, making it a logical choice when transitioning patients to longer-term oral maintenance therapy after initial use of cyclosporin for treatment-refractory or severe AD. Potential AEs include gastrointestinal side effects, fatigue, hematologic changes, and potential sequelae of immunosuppression.6,8,10 Biologics for Treatment of Atopic Dermatitis Research is in progress evaluating a variety of injectable and/or oral agents, including PDE4 inhibitors, Janus kinase (JAK) inhibitors, cannabinoid receptor agonists, kappa-opioid receptor agonists, and agents that target thymic stromal lymphopoietin (TSLP).14–17 A systematic review and meta-analysis of published studies evaluating the efficacy of biologics in AD treatment (published in April 2018) reported good evidence, to date, regarding agents that inhibit IL-4 and/or IL-13; a relative lack of evidence supporting efficacy in AD was noted thus far in studies with biologics modulating other targets, such as omalizumab (anti-IgE), infliximab ((anti-tumor necrosis factor), ustekinumab (anti-IL-12/23), and rituximab (anti-B-cell).19 IL-4 and IL-13 are reported to play prominent roles in AD with inflammation in skin and/or blood, epidermal barrier impairment, pruritis, and susceptibility to infection (Figure 1).18 Monoclonal antibodies that inhibit the effects of various ILs (i.e., IL-4, IL 13, IL-5, IL-17, IL-22, IL-31, IL-33) are showing therapeutic promise for the treatment of AD. Monoclonal Antibody Interleukin-4 and Interleukin-13 Inhibitor Dupilumab. Dupilumab is an injectable human IgG4 monoclonal antibody that inhibits IL-4 and IL-13 cytokine responses, including the expression and/or release of proinflammatory cytokines, chemokines, and IgE; binding of dupilumab occurs with both Types I and II IL-4 alpha receptors, found on hematopoietic cells and keratinocytes, respectively.13,20,21 In March 2017, duplimab was FDA-approved for the treatment of moderate-to-severe AD in adult patients (aged ?18 years) in whom the disease has not been adequately controlled with prescription topical therapies or in cases where such therapies are not advisable. In October 2018, duplimab was also approved as an add-on maintenance treatment in adolescent and adult patients (aged ?12 years of age) for moderate-to-severe asthma with an eosinophilic phenotype or oral–corticosteroid-dependent asthma.13 13 The dosing regimens for AD and asthma might differ between patients; however, the common regimen includes a 600mg loading dose (2×300mg2/mL injections), followed by a single 300mg injection every two weeks; with regard to asthma, dupilumab is not indicated or recommended for relief of acute bronchospasm or status asthmaticus.13 Clinical response. In the pivotal randomized, controlled trials (RCTs) evaluating dupilumab for AD, which included a Phase II, dose-ranging study, two 16-week monotherapy RCTs versus placebo, and a 52-week RCT that allowed for combination use with a topical CS, 1,472 subjects received dupilumab, with 739 treated for more than 52 weeks.13,20–22 Efficacy was substantiated by improvements in several assessment parameters versus placebo, both clinically and statistically, including positive changes in Investigator Global Assessment (IGA), marked reductions in Eczema Area Severity Index (EASI) scores, and significant decreases in pruritus, with clinical improvements sustained in the 52-week study without any loss of efficacy.13,20,21 Many patients reported a definite improvement in eczema and pruritus within the first few injections of dupilumab; however, onset of efficacy occurred later in some individuals (within 2 to 3 months after starting therapy). In patients currently undergoing other systemic therapies for severe AD (e.g., cyclosporin, methotrexate) who are starting dupilumab, researchers recommended that therapy be bridged without abrupt discontinuation of the patients’ previous therapy in order to avoid rebound exacerbation of AD while waiting for the clinical effects of dupilumab to manifest. Clinicians should then determine, on a case-by-case basis, the optimal approach to take when tapering patients off previous systemic therapy. 13,20–22 Safety. During the RCTs, no significant changes occurred in laboratory test results of the study subjects; thus, laboratory monitoring was not required by the FDA to be included in the approved product labeling for dupilumab.13 The most common AEs observed in the RCTs were injection site reactions and conjunctivitis (10–16% in active arms vs. 2–9% in placebo arms); separately, hypersensitivity reactions (e.g., urticaria, serum sickness-type reactions) were observed in less than one percent of the active-treatment study subjects.13,20–22 Most cases of conjunctivitis did not require stopping dupilumab, and were treated with topical ophthalmic lubricants and anti-inflammatory agents, and appeared to resolve or markedly improve despite continued use of the drug; however, some cases were severe enough to require discontinuation of dupilumab therapy.13,20–23 New onset or worsening ocular symptoms warrant referral to an ophthalmologist for evaluation.13,23 Ocular abnormalities inherent to AD that are unrelated to dupilumab use, including conjunctivitis and blepharitis, are not uncommon; the cause of the conjunctivitis that occurs related to use of dupilumab is not fully understood.24 Dupilumab and concomitant systemic therapy. A complete review of publications on dupilumab are beyond the scope of this article; however, a few articles provide information on the effective and safe use of dupilumab in a subpopulation of patients previously treated with cyclosporin. In a 16-week RCT study of adults with AD (N=390), responses to dupilumab therapy in conjunction with a medium-potency topical CS were assessed in subjects with inadequate response to or intolerance of oral cyclosporin or those in whom it was clinically inadvisable to use cyclosporin.25 Researchers reported that, following individual clinical assessment, topical CS therapy was safely tapered and/or stopped in many patients. Results of the study indicate that dupilumab with concomitant topical CS therapy (when needed) might signi?cantly improve signs and symptoms of AD and patient quality of life, with no new safety signals noted by the investigators.25 Infection risk. Eight RCTs that assessed outcomes with dupilumab versus placebo in patients with AD were analyzed by meta-analysis, with an emphasis on the incidence of AEs.26 Regarding infection rate risks, dupilumab had a lower risk of skin infection (risk ratio: 0.54), compared with placebo, with similar to negligible risks noted for nasopharyngitis, urinary tract infection, upper respiratory tract infection, and herpes virus infection. These observations further support the concept that dupilumab is immunomodulatory through the mitigation of IL-4 and IL-13 signaling, without a significant increased risk of infection, which can occur with immunosuppressive agents. It is important to note that by counteracting certain immune dysfunctions that lead to epidermal barrier impairment and cascades of Th2-driven humoral and cutaneous inflammation, dupilumab might help to normalize certain immunologic processes that are dysregulated in AD. Continued research and pharmacovigilance will help elucidate the efficacy and safety factors associated with dupilumab in greater detail. Monoclonal Antibody Interleukin-13 Inhibitors Lebrikizumab. Lebrikizumab is an injectable monoclonal antibody that exhibits high-affinity binding to soluble IL-13, thus preventing pro-inflammatory signaling by inhibiting heterodimerization of the IL-13 alpha/IL-4 alpha complex.27 In a preliminary Phase II, dose/frequency-ranging 12-week RCT, 209 adults with moderate-to-severe AD were treated with one of three dosing regimens of active drug versus placebo. Following a two-week “run in” with medium-potency topical CS therapy (triamcinolone acetonide 0.1% applied twice daily with lower potency hydrocortisone 2.5% allowed for facial AD), patients were randomized to receive lebrikizumab 125mg every four weeks, a single dose of lebrikizumab (125mg or 250mg), or placebo. Primary efficacy endpoint was the percent of subjects achieving a 50-percent reduction in EASI at Week 12.27 Investigators reported that patients in the lebrikizumab 125mg every four weeks achieved markedly superior results compared with those in the single-dose lebrikizumab group and those in the control group. Superiority to placebo was also observed in other parameters (e.g., SCORAD-50, reduction in BSA). An increasing trajectory of favorable response based on the EASI-50 results was noted at the end of the study (12 weeks) in the group receiving lebrikizumab 125mg every four weeks. Overall, the safety profile was favorable in all study arms.27 Data from this early study in AD suggest that lebrikizumab for AD shows promise as a treatment for AD. Additional research is needed on whether further increases in the dose per injection or treatment frequency (i.e., interval between doses) and use of a loading dose improve lebrikisumab’s efficacy, without affecting safety, for initial and maintenance therapy for AD. Tralokinumab. Tralokinumab, an IgG4 human monoclonal antibody that specifically neutralizes IL-13, was evaluated in a Phase IIb, dose-ranging, 12-week RCT of adult subjects (N=202) with moderate-to-severe AD.28,29 Patients were randomized to receive a 45mg (n=50), 150mg (n=51), or 300mg (n=51) subcutaneous injection of tralokinumab or placebo (n=50) every two weeks after a two-week “run in” with a mid-strength topical CS.29 Several efficacy parameters were assessed, with the coprimary endpoints being the change from baseline in total EASI score at Week 12 and the percent of IGA responders at Week 12 versus baseline (IGA score of clear/almost clear + at least a 2-grade reduction). Overall, AEs were generally similar among all study arms. Interestingly, six of the 204 subjects (2.9%) exhibited treatment-emergent conjunctivitis during the study (placebo, n= 2 [3.9%], tralokinumab 45mg, n =1 [2.0%], and tralokinumab 150mg, n=3 [5.9%]). Another important observation was that the serum level of dipeptidyl peptidase 4 might serve as a predictive biomarker for patients who could benefit from tralokinumab therapy.29 As with lebrikizumab, initial results with this agent for AD are encouraging and hopefully will be further supported by additional RCTs. Summary Points AD is a chronic disorder that, from the outset, requires a management strategy designed to control flares, decrease recurrences, and reduce pruritus. Cases of AD that are not adequately controlled with conventional measures and topical therapy can usually be effectively treated with incorporation of systemic therapy. It is important to assess the benefits versus the risks of various options in each case. It is also important to avoid becoming dependent on the intermittent use of intramuscular and/or oral corticosteroid therapy to control flares. Incorporation of other treatment options that can more optimally control AD over time are recommended. With the use of oral immunosuppressive agents such as cyclosporin, methotrexate, mycophenolate mofetil, and azathioprine, baseline and periodic laboratory and clinical monitoring are very important. Each of these agents carries its own significant “side effects baggage” to keep track of with relevant testing. Dupilumab is a newer option shown to be effective in markedly decreasing signs and symptoms of AD. In the opinion of the author, based on the available data and experiences thus far, dupilumab therapy offers a more favorable overall safety profile in comparison with the available oral systemic agents. Lebrikizumab and tralokinumab, both inhibitors of IL-13, are currently under development and show promise based on preliminary studies in adult patients with moderate-to-severe AD. References Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2—management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–132. Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 4—prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014;71(6):1218–1233. Del Rosso JQ, Harper J, Kircik L, et al. Consensus recommendations on adjunctive topical management of atopic dermatitis. J Drugs Dermatol. 2018;17(10):1070–1076. Czarnowicki T, Krueger JG, Guttman-Yassky E. Novel concepts of prevention and treatment of atopic dermatitis through barrier and immune manipulations with implications for the atopic march. J Allergy Clin Immunol. 2017;139(6):1723–1734. Thomson J, Wernham AGH, Williams HC. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a critical appraisal. Br J Dermatol. 2018;178(4):897–902. Prezzano JC, Beck LA. Long-term treatment of atopic dermatitis. Dermatol Clin. 2017;35(3):335–349. Admani S, Eichenfield LF. Atopic dermatitis. In: Lebwohl MG, Berth-Jones J, Heymann WR, Coulson I, Eds. Treatment of Skin Disease: Comprehensive Therapeutic Strategies. 4th edition. Philadelphia, PA: Elsevier-Saunders; 2014: 52–60. Akhavan A, Rudikoff D. Systemic agents for the treatment of atopic dermatitis. In: Rudikoff D, Cohen SR, Scheinfeld N (eds). Atopic Dermatitis and Eczematous Disorders. Boca Raton, FL: CRC Press/Taylor & Francis Group; 2014:187–199. Dhadwal G, Albrecht L, Gniadecki R, et al. Approach to the assessment and management of adult patients with atopic dermatitis: a consensus document. section IV: treatment options for the management of atopic dermatitis. J Cutan Med Surg. 2018;22(1 Suppl):21S–29S. Mayba J, Gooderham M. Oral agents for atopic dermatitis: current and in development. In: Yamauchi PS (ed). Biologic and Systemic Agents in Dermatology. Cham, Switzerland: Springer International Publishing; 2018:319–330. Wolverton SE. Systemic corticosteroids. In: Wolverton SE (ed). Comprehensive Dermatologic Drug Therapy, 3rd edition. Philadelphia, PA: Elsevier-Saunders; 2013:143–168. Thomas K, Bath-Hextall F, Ravenscroft J, et al. Atopic eczema. In: Williams H. Bigby M, Diepgen T, et al (eds). Evidence-Based Dermatology, 2nd edition. Malden, MA: Blackwell Publishing; 2008: 128–163. Regeneron Pharmaceuticals and Sanofi-Genzyme. Dupixent (dupilumab) Injection, Full Prescribing Information. October 2018. Kusari A, Han AM, Schairer D, et al. Atopic dermatitis: new developments. Dermatol Clin. 2019;37(1):11–20. Patel N, Strowd LC. The future of atopic dermatitis treatment. Adv Exp Med Biol. 2017;1027:185–210. Edwards T, Patel NU, Blake A, et al. Insights into future therapeutics for atopic dermatitis. Expert Opin Pharmacother. 2018;19(3):265–278. Napolitano M, Marasca C, Fabbrocini G, et al. Adult atopic dermatitis: new and emerging therapies. Expert Rev Clin Pharmacol. 2018;11(9):867–878. Silverberg JI, Kantor R. The role of interleukins 4 and/or 13 in the pathophysiology and treatment of atopic dermatitis. Dermatol Clinic. 2017;35(3):327–334. Snast I, Reiter O, Hodak E, et al. Are biologics efficacious in atopic dermatitis: a systematic review and meta-analysis. Am J Clin Dermatol. 2018;19(2):145–165. Simpson EL, Bieber T, Guttman-Yassky E, et al. Two Phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–2348. Gooderham MJ, Hong HC, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3S1):S28–S36. Hajar T, Hill E, Simpson E. Biologics for treatment of atopic dermatitis. In: Yamauchi PS (ed). Biologic and Systemic Agents in Dermatology. Cham, Switzerland: Springer International Publishing; 2018: 309–317. Treister AD, Kraff-Cooper C, Lio PA. Risk factors for dupilumab-associated conjunctivitis in patients with atopic dermatitis. JAMA Dermatol. 2018;154(10):1208–1211. Thyssen JP, Toft PB, Halling-Overgaard AS, et al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77(2):280–286. de Bruin-Weller M, Thaci D, Smith CH, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFE). Br J Dermatol. 2018;178(5): 1083–1101. Ou Z, Chen C, Chen A, et al. Adverse events of Dupilumab in adults with moderate-to-severe atopic dermatitis: a meta-analysis. Int Immunopharmacol. 2018;54:303–310. Simpson E, Flohr C, Eichenfield LE, et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with severe moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo-controlled phase II trial (TREBLE). J Am Acad Dermatol. 2018;78(5):863–871. May RD, Monk PD, Cohen ES, et al. Preclinical development of CAT-354, an IL-13 neutralizing antibody, for the treatment of severe uncontrolled asthma. Br J Pharmacol. 2012;166(1):177–193. Wollenberg A, Howell MD, Guttman-Yassky E, et al. A Phase 2b dose-ranging efficacy and safety study of tralokinumab in adult patients with moderate to severe atopic dermatitis (AD). Poster presentation. Orlando, FL: American Academy of Dermatology Meeting. 3–7 Mar 2017. Tags: Atopic Dermatitis, calcineurin inhibitors, immunosuppressants, interleukin-13, interleukin-4, phosphodiesterase-4 inhibitors Category: Atopic Dermatitis, Past Articles, What's New in the Medicine Chest
Via Krishan Maggon

|
Scooped by
Gilbert C FAURE
December 18, 2018 1:07 PM
|
Abstract The potential of precision medicine in allergy and asthma has only started to be explored. A significant clarification in the pathophysiology of rhinitis, chronic rhinosinusitis, asthma, food allergy and drug hypersensitivity was made in the last decade. This improved understanding led to a better classification of the distinct phenotypes and to the discovery of new drugs such as biologicals, targeting phenotype‐specific mechanisms. Nevertheless, many conditions remain poorly understood such as non‐eosinophilic airway diseases or non‐IgE–mediated food allergy. Moreover, there is a need to predict the response to specific therapies and the outcome of drug and food provocations. The identification of patients at risk of progression towards severity is also an unmet need in order to establish adequate preventive or therapeutic measures. The implementation of precision medicine in the clinical practice requires the identification of phenotype‐specific markers measurable in biological matrices. To become useful, these biomarkers need to be quantifiable by reliable systems, and in samples obtained in an easy, rapid and cost‐efficient way. In the last years, significant research resources have been put in the identification of valid biomarkers for asthma and allergic diseases. This review summarizes these recent advances with focus on the biomarkers with higher clinical applicability. Highlights The implementation of precision medicine in allergic diseases requires a further clarification of disease phenotypes and endotypes allowing the identification of valid biomarkers. Many of the biomarkers of allergic diseases identified to date still require validation in larger cohorts and distinct geographical areas. Multidimensional approaches have a greater potential to identify valid biomarkers for allergic and chronic respiratory diseases. 1 INTRODUCTION Precision medicine for allergic diseases requires a deep understanding of immunopathology and phenotype heterogeneity in relation to clinically significant outcomes.1 Precision medicine could also help to limit the socio‐economic burden imposed by allergic and chronic respiratory diseases.2 According to the National Institutes of Health (NIH), precision medicine is an emerging approach for disease treatment and prevention that takes into account individual variability in genes, environment and lifestyle for each person. In this regard, asthma and allergic conditions are ideally suited, as they represent umbrella entities comprising different diseases partially sharing immune mechanisms (endotypes) and presenting similar visible properties (phenotypes), but requiring individualized approaches for a better risk prediction and the identification of treatment responders.3 The implementation of precision medicine demands measurable indicators of biological conditions usually termed biomarkers. A valid biomarker should be quantifiable in an analytical system with well‐defined performance and need to be supported by a body of evidence which sufficiently clarifies the pathological and clinical significance of the test results.4 Moreover, the identification of novel biomarkers applicable in daily practice requires clear clinical models with well‐established extreme phenotypes, allowing a better understanding of the disease progression along its severity. Another aspect influencing the clinical applicability is the biological matrix (“sample type”) where biomarkers are measured (Table 1). Appropriate matrices should be easy to obtain, store and manipulate in standardized and reproducible measuring protocols at a reasonable cost. Moreover, in most cases a single biomarker will not adequately represent the complexity of mechanisms underlying multifactorial diseases. In this regard, the generation of multidimensional biomarker panels displays a greater potential to identify valid markers.5 The ideal biomarker Supported by a body of evidence clarifying its biological significance Quantifiable in a cost‐efficient analytical system with well‐defined performance Detectable in a biological matrix obtained in an easy, rapid and cost‐efficient way The research efforts in asthma and allergic diseases during the last decades have focused on the identification of biomarkers applicable in clinical practice. Although several markers of allergic inflammation (e.g, IgE, eosinophilia, fractional exhaled nitric oxide [FENO]) have been described, their utility in diagnosis, prognosis and therapy is still controversial.6-8 Different types of molecules (genes, metabolites, etc.) have been also proposed as biomarkers for allergic and chronic respiratory conditions. Some of them display good analytical properties, but overall, they are insufficiently robust to be extrapolated to clinical practice. This fact partially arises from a paucity of clinical models for allergic diseases, which clearly constitutes a limiting factor in the search for biomarkers. This review will summarize the biomarkers identified to date for allergic and chronic respiratory conditions, with special focus on those with higher clinical applicability. 2 NEW METHODS TO IDENTIFY BIOMARKERS: THE OMICS The different omics characterize and quantify biological molecules, which share a common feature and which provide information about the structure and function of organisms. Omics are significantly contributing to the definition of disease endotypes and phenotypes and to the identification of therapeutic targets. Recent technological advances have pushed the omics field forward by allowing higher throughputs, improving detection limits and providing software tools to analyse and visualize the data. Genomics, transcriptomics and epigenetics have been used to identify genes, RNA sub‐types and DNA modifications, respectively. Nevertheless, the validation of these observations requires the investigation of their functional consequences (e.g, the effect in transcription or splicing variants or in the functionality of proteins). Only this step permits valid conclusions for the underlying mechanisms of disease phenotypes (Figure 1). Metabolomics investigates the nature and concentration of the metabolites generated in living systems and is among the most recent approaches applied to allergy research. Metabolomics displays a high degree of versatility, as it is applicable to a great variety of matrices, whose nature can be tailored to the disease of interest.4, 7 In chronic respiratory diseases, the metabolic analysis of exhaled breath condensate seems a promising matrix for biomarker identification. Other approaches which could help phenotype chronic airway conditions include electronic nose (eNose) or nuclear magnetic resonance–based metabolomics.7 Nevertheless, the lack of standardized procedures for breath sampling, the effect of pH on some metabolites and the low concentrations are well‐established limiting factors. Individual omics display both strengths and weaknesses which overall define the validity and robustness of the results. The development of multi‐omics and multi‐matrix platforms in integrated approaches will probably provide a more holistic picture of biological situations, including allergic and chronic respiratory diseases. Nevertheless, the implementation of these advances in clinical practice requires the identification of biomarkers measurable in biological fluids obtained in an easy, rapid and cost‐efficient way. 3 UPPER AND LOWER AIRWAY DISEASES 3.1 Rhinitis Chronic rhinitis is generally divided into allergic rhinitis (AR) and non‐allergic rhinitis (NAR).9 The NAR category comprises a heterogeneous group of diseases mediated by immune or neurogenic mechanisms.10 Conversely, AR is a relatively homogeneous entity arising from IgE‐mediated inflammation.11 Local allergic rhinitis (LAR) is a disease phenotype not fitting into the AR‐NAR dichotomy.12 The skin prick test (SPT) and/or serum allergen–specific (s)IgE and the nasal allergen challenge (NAC) positively identify AR patients. Subjects with LAR are defined by a positive NAC with negative SPT and serum sIgE, whereas NAR patients test negative for the three biomarkers. Several inflammatory cells and mediators may also serve as diagnostic biomarkers for AR. Eosinophils, IL‐5, IL‐6, IL‐13 and macrophage inflammatory protein (MIP)‐1β increase in the nasal lavage of AR patients following NAC,13 while elevated nasal endothelin (ET)‐1 and CCL17 at baseline discriminate AR from NAR individuals.14 Compared to healthy controls, subjects with house dust mite (HDM)–induced AR have increased circulating group 2 innate lymphoid cells (ILC2), which also correlate with serum IL‐13 and symptom scores.15 Allergen immunotherapy (AIT) is an effective treatment for AR16 and LAR17, 18 but involves considerable time and cost. Biomarkers assisting the selection of patients most likely to respond to AIT have recently been summarized.16 A proportion of sIgE to total IgE >16.2% predicted AIT success with 97.2% sensitivity and 88.1% specificity.19 In children, low serum osteopontin identifies responders to sublingual immunotherapy.20 Serum osteopontin and basophil reactivity increase after NAC21 and diminish following successful AIT.16 Moreover, subcutaneous immunotherapy with HDM armed peripheral T regulatory cells with the ability to inhibit Th2 and Th9 proliferation.22 3.2 Chronic rhinosinusitis Chronic rhinosinusitis (CRS) is defined by nasal and sinus mucosal inflammation. Phenotyping of CRS is typically based on the presence (CRSwNP) or absence (CRSsNP) of nasal polyps on endoscopy or CT scan, whereas examination of nasal samples facilitates the endotyping (Figure 2). In Caucasian populations, nasal polyps generally show an eosinophilic infiltrate, whereas fewer than 50% of Asian patients display eosinophilic polyps.23, 24 One study identified ten CRS clusters, of which six exhibited a type 2 inflammatory profile with raised IL‐5 and eosinophilia. Type 2 clusters displayed a higher risk of nasal polyps and asthma.25 The CRSwNP phenotype has been also associated with increased ILC2 at both the tissue level and peripheral blood.26, 27 Several matrices have been used to predict CRS prognosis. Blood and tissue eosinophilia correlates with severity as measured by endoscopy and CT scan28 and can predict recurrence following endoscopic sinus surgery.23 In addition, a small study observed that programmed cell death‐1 (PD‐1) mRNA expression in nasal polyp tissue correlated with disease severity on CT scan,29 while tissue gene expression of the eosinophil marker—Charcot‐Leyden crystal protein—was associated with higher olfactory impairment.30 The level of IL‐5 and P‐glycoprotein in nasal secretions helped to predict the olfactory and the CT scan scores, respectively.31 Nasal nitric oxide (nNO) inversely correlated with CT scan‐graded severity and increased after sinus surgery.32 3.3 Asthma Asthma phenotypes are classified into those displaying dominant type 2 inflammation, and those without significant type 2 inflammation,33 with each group comprising a number of different diseases (Figure 3). Several biomarkers measurable in different matrices have been described for these asthma phenotypes. The relevance of asthma endotyping is perfectly illustrated by the case of the anti‐IL‐5 monoclonal antibody (mAb) mepolizumab whose initial lacklustre performance in unclassified asthma34 was followed by excellent outcomes when administered to patients with eosinophilic asthma.35 In any individual, the disease expression may be driven by complex endotypes with numerous mechanistic pathways3; therefore, multidimensional biomarker assessment may be required. Unsupervised statistical analyses examining various blood inflammatory mediators identified unique clusters with different clinical and pathological features. Cluster analysis using sputum mediators at exacerbation also identified distinct biologic clusters with differences in host microbiome. The subsequent paragraphs describe individual biomarkers according to their biological matrices in more detail. 3.4 Diagnostic biomarkers 3.4.1 Blood cells Peripheral blood eosinophilia and neutrophilia in asthma have been associated with different clinical characteristics, with neutrophilia indicating increased sputum production.36 In asthma, neutrophilia is also more prevalent among patients with a smoking history and persistent airflow limitation compared to non‐smoking asthma patients (4.5 × 109 vs 3.6 × 109 cells/L),37 suggesting that neutrophilia may differentiate between patients with asthma alone from those with features of asthma‐chronic obstructive pulmonary disease (COPD) overlap (ACO) syndrome. Several genes regulating immune cells such as B lymphocytes, T lymphocytes and granulocytes were up‐ or down‐regulated in severe asthma compared to healthy controls.38 In a paediatric asthma cohort, the expression of five selected genes on CD4 lymphocytes (SRM, HDAC2, SLC33A1, P2RY10 and ADD3) predicted the atopic status with 100% sensitivity and 81.3% specificity,39 while in another paediatric study, a fourteen‐gene signature (MCEMP1, AQP9, PGLYRP1, S100P, RNASE2, OLFM4, CAMP, CEACAM8, LCN2, MPO, DEFA4, ELANE, BPI, DEFA1B, CTSG, HBD, ALAS2, RPS4Y2 and RPS4Y1) was unique to a neutrophilic phenotypic cluster.40 In a recent pilot study, circulating blood microRNA profile (expressed as miRNA ratios) showed promise in differentiating allergic asthma from healthy controls.41 Lipidomic profile and gene expression after low molecular weight hyaluronic acid stimulation of peripheral blood mononuclear cells were also shown to be different in severe asthma compared to mild asthma and healthy controls.42 Peripheral differential cell counts can also serve as surrogate markers of airway inflammation. In a meta‐analysis of 14 studies, the ability of blood eosinophils to predict airway eosinophilia showed an area under the curve (AUC) of 0.78.43 Enumeration of peripheral ILC2 has a similar utility for predicting sputum eosinophilia.44 Conversely, blood neutrophilia is less indicative of sputum neutrophilia, with an AUC of only 0.6.45 The ex vivo response of blood neutrophils and eosinophils to stimulation with N‐formyl‐methionyl‐leucyl‐phenylalanine (fMLP), in combination with relevant clinical parameters, is also able to predict sputum eosinophilia.46 3.4.2 Serum mediators The chitinase‐like protein YKL‐40 distinguished asthma from COPD and healthy controls.47 Neutrophil expression of Siglec‐9 is increased in patients with COPD and may have future potential to diagnose asthma.48 Serum (soluble‐cleaved) urokinase plasminogen–activated receptor (scuPAR) was found to be higher in severe, non‐atopic asthma in a single study49 and requires confirmation in further studies. Blood mediators can also predict airway inflammation. Overall, serum periostin moderately correlated with sputum eosinophilia.50, 51 Eosinophilic cationic protein (ECP) is more predictive of sputum eosinophilia than serum IgE,39 whereas C‐reactive protein (CRP) is weakly associated with sputum neutrophilia.52 3.4.3 Sputum cells and mediators Sputum quantitative cell count is a reference standard for airway inflammation in asthma. Four inflammatory phenotypes have been described—eosinophilic, neutrophilic, mixed granulocytic and paucigranulocytic. The neutrophilic subtype has been associated with the obese female asthma non‐type 2 phenotype.52 The Unbiased Biomarkers for the Prediction of Respiratory Disease Outcomes (UBIOPRED) study is a multicentre prospective cohort study recruiting patients with severe asthma in various European countries. Sputum analysis of the UBIOPRED cohort identified 3 transcriptome‐associated clusters (gene clusters), corresponding to eosinophilic, neutrophilic and paucigranulocytic phenotypes, respectively.53 A six‐gene signature (CLC, CPA3, DNASE1L3, IL1B, ALPL and CXCR2) can differentiate asthma patients from controls and distinguish between eosinophilic from neutrophilic asthma.54 This method has a practical advantage over sputum differential cell counts, as frozen samples can be batched for processing. Neutrophil myeloperoxidase in sputum has the potential to differentiate ACO from asthma.55 Specific microRNAs can discriminate neutrophilic from eosinophilic asthma.56 Sputum eosinophil peroxidase (EPX) correlates with sputum eosinophilia,57 as does nasal and pharyngeal EPX.58 Importantly, nasal sampling may be particularly useful for patients in whom sputum induction is unsafe or not possible. 3.4.4 Cellular bronchial samples Patients in the UBIOPRED cohort could be divided into four groups based on the expression of nine gene sets in bronchial cells, each with mixed inflammatory patterns including one with concomitant Th2 and Th17 markers.53 Interestingly, a different study found that Th2 and Th17 gene expression signatures were mutually exclusive in asthmatic airway tissue.59 The authors suggested that suppression of Th2 activity by corticosteroids may accentuate Th17 activity. Different gene signatures for Th2 and Th17 activities were used in the two studies, possibly limiting direct comparisons. The interplay between Th2 and Th17 cells is also likely to be complex, and the exact relationship between the two is still uncertain.60 3.4.5 Exhaled breath The FENO displays an AUC of 0.8 for asthma diagnosis.2 Of note, very high or low cut‐offs for FENO can, respectively, rule‐in or rule‐out asthma.27 Conversely, FENO has limited utility to predict sputum eosinophilia,43 as it is confounded by corticosteroid treatment, atopy and smoking status. Volatile organic compounds (VOCs) in exhaled breath can be readily measured using eNose devices. Building on previous work which discriminates between COPD and asthma, eNose has identified label‐free clinical and inflammatory clusters among asthma and COPD patients.61 VOCs and other metabolites in exhaled breath can also differentiate asthma from healthy controls in adults and children.62-65 In a paediatric study, metabolomic analysis using nuclear magnetic resonance (NMR) of exhaled breath condensate identified three clusters with different inflammatory profiles based on global spectral patterns of NMR.66 3.4.6 Urine Urine metabolite analysis can accurately discriminate between asthma and COPD67 and also correlates with FENO and blood eosinophilia.68 3.5 Prognostic biomarkers 3.5.1 Blood cells Raised blood eosinophils strongly predict the risk of asthma exacerbations in both adults and children.69, 70 Blood eosinophilia also predicts longitudinal lung function decline, irrespective of smoking status.71 Blood neutrophilia is linked with airway infections in asthma11 as well as poor symptom control and increased exacerbations.72 Circulating blood fibrocytes correlate with asthma severity.73 3.5.2 Serum mediators The stability of serum periostin over disease progression facilitates its use as a biomarker.74 Elevated levels are associated with fixed and more severe airflow obstruction75, 76 and greater longitudinal lung function decline.77 Total serum IgE in children is associated with atopy, airway hyperresponsiveness (AHR) and bronchial wall thickening in CT scan.69 In both adults and children, YKL‐40 level correlates with severe asthma and poor lung function.78, 79 The expression of ten selected microRNAs (HS_108.1, HS_112, HS_182.1, HS_240, HS_261.1, HS_3, HS_55.1, HS_91.1, hsa‐miR‐604 and hsa‐miR‐638) was higher in children with severe asthma.80 3.5.3 Sputum cells and mediators Sputum neutrophilia and ILC2 27 are associated with asthma severity.81, 82 Changes in sputum eosinophilia reflect fluctuations in clinical asthma control.83 Human tumour necrosis factor–like weak inducer of apoptosis (TWEAK) is an inflammatory mediator whose level in sputum correlated with higher severity, poor symptom control and decreased lung function in children with non‐eosinophilic asthma.84 3.5.4 Cellular bronchial samples Bronchial neutrophilia is present in severe (compared to non‐severe) asthma, independent of oral corticosteroid (OCS) intake.85 Gene signatures analysed in endobronchial brushing and biopsy specimens predicted persistent airflow limitation in the UBIOPRED cohort.86 In bronchoalveolar lavage samples, elevated CD4+ cells expressing both IL‐4 and IL‐17 predicted greater asthma severity.69, 87 3.5.5 Exhaled breath In both children and adults, FENO correlates with greater AHR, airway obstruction and exacerbations.69, 88 Patients with FENO >45 ppb are at greater risk for suffering >2 asthma exacerbations/year.89 Electronic nose‐measured VOCs predicted the loss of asthma control upon withdrawal of inhaled corticosteroids (ICS).90 Reactive oxygen species (ROS) can also be detected in exhaled breath condensates of patients with asthma and has been shown to be suppressed by anti‐inflammatory agents.91 3.5.6 Functional imaging of lungs Functional imaging with hyperpolarized gas magnetic resonance of the lung can predict asthma outcomes; persistent ventilation defects were associated with poorer asthma control.92 Greater ventilation defects are also observed in patients with uncontrolled eosinophilic inflammation.93 3.6 Biomarkers for therapeutic response prediction and measurement 3.6.1 Blood cells Blood eosinophilia identifies asthma patients responding to therapies targeting type 2 inflammation. The post hoc analyses of randomized controlled trials with the anti‐IgE mAb omalizumab identified blood eosinophilia (≥300 cells/μL) as a predictor of greater response.94, 95 Nevertheless, this finding was not reproduced in a real‐life study.96 There is a direct correlation between blood eosinophilia and the response to mepolizumab,97 the anti‐IL‐5 receptor mAb benralizumab98 and the anti‐IL‐4 receptor mAb dupilumab.99 Blood eosinophilia may also predict and monitor the response to corticosteroids. Atopic children with eosinophilia ≥300 cells/μL respond better to ICS.100 A decrease in peripheral eosinophilia is observed with the up‐dosing of ICS,101 while titration of OCS to maintain blood eosinophilia <200 cells/μL improved asthma control.102 3.6.2 Serum mediators Elevated serum periostin predicts the response to omalizumab.75, 103 Interestingly, total serum IgE does not predict the response to omalizumab, despite this molecule being not only the drug target, but also the basis for its dose calculation.104 On the other hand, a reduction in serum‐free IgE after 16‐32 weeks on omalizumab is associated with a decrease in exacerbations over two years.81 3.6.3 Sputum Sputum eosinophilia ≥3% predicts response to corticosteroids105 and mepolizumab.35 Sputum eosinophilia as a guide for ICS therapy reduced exacerbations with no associated increase in the total ICS dose.106, 107 3.6.4 Exhaled breath In patients with symptoms suggestive of AHR, elevated FENO predicts response to ICS.108 A systematic review concluded that using FENO to guide ICS therapy in adults reduced the mild but not the severe exacerbations.109 Among children, FENO also showed unclear benefits on asthma outcomes.110 A FENO level >19.5 ppb also correlated with response to omalizumab.75 3.6.5 Urine Urine bromotyrosine correlates with corticosteroid responsiveness, and the predictive accuracy further improves when combined with high FENO levels.94 Despite the previous enumeration being made in a matrix‐related fashion, the complexity of most asthma phenotypes will require multidimensional approaches to identify valid biomarkers (Table 2). This aspect is exemplified by the greatest benefit from dupilumab being observed in asthma patients exhibiting both elevated peripheral eosinophilia and FENO.99 Diagnosis Prognosis Response prediction and monitoring Blood cells Distinguish asthma from COPD Blood neutrophil Distinguish asthma from healthy controls Gene expression Inflammatory phenotyping Blood eosinophils Blood neutrophils Responsiveness of blood neutrophils and eosinophils to fMLF Exacerbations Eosinophils Neutrophils Symptoms Neutrophils Lung function Eosinophils Asthma severity Fibrocytes Predict response to anti‐IL‐5 Eosinophils Predict response to ICS Eosinophils Monitor response to corticosteroids Eosinophils Blood mediators Distinguish asthma from COPD YKL‐40 Siglec‐9 Determine atopy status scuPAR Inflammatory phenotyping ECP CRP Lung function Periostin YKL‐40 Airway remodelling IgE Asthma severity IgE MicroRNA YKL‐40 Predict response to omalizumab Periostin Reduction in serum‐free IgE Sputum cells Inflammatory phenotyping Quantitative cell count Gene signature Lung function Neutrophils Asthma severity ILC2 Loss of asthma control Eosinophils Predict response to mepolizumab Eosinophils Predict response to corticosteroids Eosinophils Guide ICS titration Eosinophils Sputum mediators Distinguish asthma from COPD MPO Inflammatory phenotyping MicroRNA Sputum/nasal/pharyngeal EPX Asthma severity TWEAK Bronchial tissue Inflammatory phenotyping Gene expression Lung function Gene signatures Asthma severity Neutrophils Exhaled breath Asthma diagnosis FENO VOC Metabolites Inflammatory phenotyping eNose Exacerbations FENO Lung function FENO Loss of asthma control eNose Predict response to ICS FENO Guide ICS therapy FENO (in adults) Urine Distinguish asthma from COPD Metabolites Inflammatory phenotyping Urine metabolites Predict response to corticosteroids Urine bromotyrosine COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; ECP, eosinophilic cationic protein; eNose, electronic Nose; EPX, eosinophil peroxidase; FENO, fractional exhaled nitric oxide; fMLF, N‐formyl‐methionyl‐leucyl‐phenylalanine; ICS, inhaled corticosteroid; IgE, immunoglobulin E; IL‐5, interleukin‐5; ILC2, group 2 innate lymphoid cell; LTE4, leukotriene 4; MPO, myeloperoxidase; NERD, aspirin‐exacerbated respiratory disease; scuPAR, serum soluble‐cleaved form of the urokinase plasminogen–activated receptor; Siglec‐9, sialic acid–binding immunoglobulin‐type lectins‐9; TWEAK, tumour necrosis factor–like weak inducer of apoptosis; VOC, volatile organic compounds. 4 FOOD ALLERGY AND ANAPHYLAXIS The food allergy (FA) phenotypes differ on their IgE dependence and prognosis (Figure 4).111 Given this heterogeneity, the search for FA biomarkers has gained significant attention.112 4.1 IgE‐mediated food allergy The identification of children at risk of developing FA might help establish preventive strategies.112 The balance between type 2 and type 1 chemokines in cord blood influenced the sensitization to food allergens at the age of 3 years in children from Taiwan.113 Atopic individuals often display skin prick test (SPT) positivity to foods they tolerate.112 Indoleamine 2,3‐dioxygenase (IDO) is a tryptophan‐catabolizing enzyme expressed by antigen‐presenting cells.114 A high IDO activity was associated with unresponsiveness to food allergens in sensitized children from Turkey.114 Molecular allergology is a useful tool to identify clinically relevant IgE sensitization.2, 112 Specific (s)IgE to the storage proteins Cor a 14 from hazelnut or Ana o 1, 2 or 3 from cashew, correlated with clinically relevant sensitization in children from Denmark115 and the Netherlands,116 respectively. These observations might facilitate the management of patients with FA by limiting the number of oral food challenges (OFC) necessary for diagnosis.64, 112 Interestingly, a score based on the value of sIgE to Ana o 3, the SPT wheal size and the gender of the patient was proposed to predict the outcome of cashew OFC in Dutch patients.117 Basophil activation test (BAT) might also correlate with the OFC outcome in food‐dependent NSAID‐induced anaphylaxis.118 Anaphylaxis is the most severe phenotype of IgE‐mediated hypersensitivity, and the increase in serum tryptase is a helpful biomarker in most cases.119 In Canadian children, milk was the food most likely to increase serum tryptase levels.119 Interestingly, the combination of the serum levels of apolipoprotein A1 and the prostaglandin D2 metabolite 9α,11β‐PGF2, displayed a good diagnostic performance for food‐induced anaphylaxis in German patients.120 Oral immunotherapy (OIT) is a promising tool for persistent forms of IgE‐mediated FA.111, 121-123 In anaphylactic children from the United States, successful milkOIT induced the increase in peripheral invariant natural killer T (iNKT) cells and skewed milk‐stimulated iNKT cells from a type 2 to a type 1 profile.124 Furthermore, successful OIT reduced blood eosinophils and increased several mediators functionally related to type 1 immunity (adipokines, leptin or resistin) in milk‐allergic children from Finland.125 A higher baseline sIgA and a rapid increase in sIgG1 after OIT initiation identified good responder egg‐allergic children from Japan.126 The adverse reactions (AdR) during OIT limit its use in the clinics.123 In children undergoing peanutOIT, the presence of allergic rhinitis and the SPT wheal size were associated with systemic and gastrointestinal AdR.127 Adjuvant therapy with omalizumab might reduce AdR during OIT,123 and the combination of basophil reactivity and sIgE/total IgE ratio at baseline could identify patients more likely to benefit from omalizumab during milkOIT.128 Beyond the oral route, other administration routes are under investigation for severe FA.123 Sublingual immunotherapy with Pru p 3, the lipid transfer protein from peach, induced anti‐inflammatory PDL‐1+ dendritic cells and IL‐10+ T regulatory cells in responder patients from Spain.129, 130 4.2 Other types of food allergy The diagnosis of eosinophilic esophagitis requires the demonstration of >15 eosinophils/high‐power field in the oesophagus of individuals with suggestive symptoms.131 Oesophageal eosinophilia correlated with male gender and the number of positive food sIgE tests in American children.132 This observation might help to limit the number of endoscopies required for diagnosis.131 The management of food protein‐induced enterocolitis syndrome (FPIE) patients involves consecutive OFCs to asses for disease resolution.133 In Japanese children with FPIES, the OFCs induced the activation of intestinal and peripheral eosinophils.134 Interestingly, the peripheral level of C‐reactive protein and of eosinophilia correlated with a poor and good prognosis, respectively, in Japanese patients with FPIES.135 Despite the progress made in recent years, most biomarkers remain to be validated in larger populations and distinct geographical areas. Moreover, growing evidence suggests that airway allergy influences many of the parameters identified as FA biomarkers.136, 137 In this regard, the clarification of atopic phenotypes and their relationship with FA will improve the interpretation of biomarkers.68, 138 5 DRUG HYPERSENSITIVITY A summary of the different drug hypersensitivity phenotypes can be seen in Figure 5. 5.1 Cross‐intolerance to NSAIDs Non‐steroidal anti‐inflammatory drugs (NSAIDs) are the most common triggers of drug hypersensitivity reactions, and in most cases, these reactions are not mediated by immunological mechanisms.139 In non‐selective or cross‐intolerant reactions, NSAIDs from different groups provoke skin or respiratory symptoms.140 In these cases, the reaction‐inducing potential does not rely on the chemical structure of the drug, but on its COX‐1 inhibitory activity.140 Aspirin‐ or NSAID‐exacerbated respiratory disease (AERD and NERD, respectively) is the most studied phenotype of cross‐intolerance. This entity is defined by the onset of respiratory symptoms upon intake of NSAIDs and is related to a dysregulation of arachidonic acid (AA) metabolism with overproduction of leukotrienes (LT) and prostaglandins (PG).141 Many NERD subjects have concomitant CRS and asthma.141 In a Korean study, NERD patients were divided into four sub‐phenotypes based on the presence of CRS, urticaria and atopy.142 Interestingly, significant differences existed in asthma severity, total serum IgE, sputum and peripheral eosinophilia, and urinary LTE4 (uLTE4).142 In American patients, uLTE4 helped to identify aspirin sensitivity in patients with different nasal inflammatory conditions.143 A recent meta‐analysis reported that the sensitivity and specificity of uLTE4 for identifying aspirin sensitivity in asthma ranged from 0.55 to 0.81 and from 0.77 to 0.82, respectively, depending on the detection method.144 Serum LTE4 in combination with LTE4/PGF2α ratio might help to detect NERD among other asthma phenotypes.145 Aspirin provocation increased 8‐iso‐PGE2 in the exhaled breath condensate of NERD patients and correlated with uLTE4.146 Platelet activation was also associated with overproduction of AA metabolites and to a reduced lung function in NERD patients.147 Other biomarkers beyond AA metabolites have been related to NERD. The serum sphingosine‐1‐phosphate was higher in NERD patients than in other asthmatics.148 American patients with NERD displayed higher activation of mast cells, basophils and platelets measured in nasal microparticles than other CRS individuals.149 Overall, these biomarkers might facilitate the diagnosis of NSAID hypersensitivity by decreasing the need for drug provocations. Some cross‐intolerance phenotypes resolve over time,150 and these biomarkers might help determine the most adequate timing to test for aspirin tolerance. 5.2 Immune‐mediated reactions These conditions can be divided into immediate and non‐immediate reactions arising from IgE‐ and T cell–mediated mechanisms, respectively (Figure 5).112 5.3 Immediate reactions Betalactams (BL) and fluoroquinolones (FQ) are the most common drugs involved in immediate reactions.139 5.3.1 Betalactams Skin testing displays a diagnostic sensitivity of up to 70%.151 Available in vitro tests include immunoassays to quantify serum BL‐sIgE, including the commercial ImmunoCAP© (Thermo‐Fisher, Uppsala, Sweden).151 Its sensitivity shows a high variability (0%‐50%),152 depending on the reaction severity and the time gap at the moment of measurement.153 Moreover, ImmunoCAP© can induce false‐positive results when testing for Penicillin‐V.154 Increased serum tryptase during the acute phase of reactions can confirm mast cell activation 112 and correlates with the severity.155 The sensitivity of BAT for BL allergy ranges from 22% to 55% with a specificity of up to 96%.156, 157 5.3.2 Fluoroquinolones Skin testing is not useful for the diagnosis of FQ allergy,158 and there are no available immunoassays. The CD63‐based BAT displayed 83.3% sensitivity and 88.9% specificity for ciprofloxacin allergy.159 Surprisingly, CD203c outperforms CD63 as BAT‐activation marker for moxifloxacin allergy, yet its sensitivity was low (36.4%).159, 160 These data question the role of basophils in moxifloxacin allergy, but identify BAT as a promising tool for ciprofloxacin allergy. 5.4 Non‐immediate reactions Patch testing and intradermal test with delayed reading are useful in vivo biomarkers. The sensitivity of the in vitro lymphocyte transformation test (LTT) is lower than that of BAT for immediate reactions.63, 155 A combination of granzyme B and granulysin expression in blood cells can detect lymphocyte activation in the setting of severe cutaneous reactions like Stevens‐Johnson syndrome.161 The screening for HLAB*57:01 before abacavir prescription is recommended by regulatory agencies,162 as it showed 100% of negative predictive value for immunologically confirmed abacavir hypersensitivity.163 The screening for HLAB*15:02 is also recommended before carbamazepine treatment in patients at high risk (Han Chinese, Vietnamese, Cambodians, etc.).164, 165 6 CONCLUSIONS The potential of precision medicine in the fields of allergy and chronic respiratory diseases has only started to be explored. A better definition of disease phenotypes and endotypes based on treatable traits and other clinically significant aspects is a prerequisite to progress in individualized therapies. In the last decade, we have seen an improvement in the definition of allergic respiratory disease, and we have gained insights into other eosinophilic phenotypes of rhinitis, CRS and asthma. This knowledge has translated into a significant broadening of therapeutic options, including (but not limited to) new biologicals. Because precision medicine needs to be performed in a cost‐efficient way, there is a need to identify responder patients to these new drugs. On the other hand, the available therapies for NAR and non‐eosinophilic CRS and asthma are much more limited, reflecting the important knowledge gaps in the pathophysiology of those phenotypes. Similarly, there is a need to progress in the definition of EoE and FPIES in order to improve the clinical management of the patients. The clarification of the disease mechanisms behind IgE‐mediated food allergy and drug hypersensitivity will help identify patients at risk of developing allergic reactions, limit the number of required provocations and establish preventive strategies. Nevertheless, the clarification of disease phenotypes per se does not guarantee the implementation of precision medicine in the clinical practice, as this step requires the detection of valid biomarkers. This search will be a long and resource‐consuming path requiring large population cohorts. Among the different disciplines applied to biomarker identification, metabolomics appears as a promising tool, yet growing evidence indicates that valid biomarkers will be detected by multidimensional strategies. Valid biomarkers do not only need to accurately reflect the phenotype‐specific disease mechanisms, but also to be quantifiable in a rapid, easy and cost‐efficient way. Only under these premises, the research in biomarker identification will be able to impact the clinical practice and translate into an improved diagnosis, management, and treatment of patients with allergic and chronic respiratory diseases. ACKNOWLEDGMENTS The present work has been supported by the Institute of Health “Carlos III” of the Spanish Ministry of Economy and Competitiveness (grants cofunded by the European Regional Development Fund (ERDF): thematic network and cooperative research centres ARADyAL RD16/0006/0001 and RD16/0006/0015, and research projects PI15/02256, PI16/00249, PI17/01318. This article has been also supported by research grants provided by the Regional Ministry of Health of Andalusia: PI‐0346‐2016 and PC‐0278‐2017. I Eguiluz‐Gracia holds a Rio Hortega research contract (CM17/00140) of the Institute of Health “Carlos III,” Spanish Ministry of Economy and Competitiveness (cofounded by the European Social Fund, ESF). CONFLICT OF INTEREST None of the authors have any conflict of interest in relation to this article. REFERENCES

|
Scooped by
Gilbert C FAURE
November 8, 2018 11:10 AM
|
Omalizumab is approved for use in chronic spontaneous urticaria (CSU); however, it is not approved for chronic inducible urticaria (CIndU).The aim of the present study was to assess the effectiveness...

|
Scooped by
Gilbert C FAURE
October 4, 2018 8:13 AM
|
Learn more about urticaria, also known as hives, and angioedema; skin conditions which give wheals or swelling. Read our urticaria (hives) and angioedema factsheets for treatment tips.

|
Scooped by
Gilbert C FAURE
July 30, 2018 1:19 PM
|

|
Scooped by
Gilbert C FAURE
June 24, 2018 5:15 AM
|
This article is developed by the Skin Allergy Research Society of India for an updated evidence-based consensus statement for the management of urticaria, with a special reference to the Indian context.

|
Scooped by
Gilbert C FAURE
February 3, 2018 11:31 AM
|
Ther Clin Risk Manag. 2016 Apr 13;12:585-97. doi: 10.2147/TCRM.S105189. eCollection 2016. Review

|
Scooped by
Gilbert C FAURE
July 8, 2017 1:52 AM
|
Treatment with second-generation antihistamines is recommended in patients with chronic spontaneous urticaria (CSU). Some patients remain unresponsive even after up-dosing up to fourfold. Many third line treatment options have limited availability and/or give rise to significant side effects. We investigated effectiveness and safety of antihistamine treatment with dosages up to fourfold and higher. This retrospective analysis of patients’ records was performed in adult CSU patients suffering wheals and/or angioedema (AE). Demographic, clinical, and therapeutic data was extracted from their medical records. We recorded the type, maximum prescribed dosage, effectiveness, and reported side effects of antihistamine treatment. Of 200 screened patients, 178 were included. Treatment was commenced with a once daily dose of antihistamines. Persisting symptoms meant that up-dosing up to fourfold occurred in 138 (78%) of patients, yielding sufficient response in 41 (23%). Up-dosing antihistamines was necessary in 110 (80%) patient with weals alone or weals with angioedema and 28 (64%) with AE only (p = 0.039). Of the remaining 97 patients with insufficient response, 59 were treated with dosages higher than fourfold (median dosage 8, range 5–12). This was sufficient in 29 patients (49%). Side effects were reported in 36 patients (20%), whereof 30 (17%) experienced somnolence. Side effects after up-dosing higher than fourfold were reported in six out of 59 patients (10%). Up-dosing antihistamines higher than fourfold dosage seems a feasible therapeutic option with regards to effectiveness and safety. The need for third line therapies could be decreased by 49%, with a very limited increase of reported side effects.

|
Scooped by
Gilbert C FAURE
December 3, 2016 11:00 AM
|
Food industry workers are at increased risk for occupational contact urticaria (CU). There are many foodstuffs that have been reported to cause occupational CU, including seafood, meat, vegetables
|

|
Scooped by
Gilbert C FAURE
July 28, 2023 2:53 AM
|
📃Our latest article covers new evidence on the role of autoimmunity in chronic spontaneous urticaria.
💌Thanks to Yi-Kui Xiang, Daniel Elieh-Ali-Komi and… | 10 comentários no LinkedIn

|
Scooped by
Gilbert C FAURE
December 30, 2021 4:11 AM
|
Angioedema is a prevailing symptom in different diseases, frequently occurring in the presence of urticaria. Recurrent angioedema without urticaria (AE) can be hereditary (HAE) and acquired (AAE), and several subtypes can be distinguished, although clinical presentation is quite similar in some of...

|
Scooped by
Gilbert C FAURE
September 12, 2020 4:20 AM
|
Morning Session Morning Chair: Dr Tom Marrs 09:00 Welcome and Introduction Dr Tom Marrs Consultant in Paediatric Allergy St Thomas' Hospital, London 09:10 Remote managment of eczema and urticaria in primary care Dr Alya Abdul-Wahab Consultant Dermatologist St George's Hospital, London 09:50 How to fix common gastro-intestinal symptoms in children Dr Rakesh Vora Paediatric Gastroenterology Consultant St Thomas' Hospital, London 10:35 << BREAK >> 11:00 How to manage IgE and non-IgE food allergies Dr Tom Marrs Consultant in Paediatric Allergy St Thomas' Hospital, London 11:40 How to recomment avoidance of egg and nuts Rebecca Brocklehurst Paediatric Allergy Dietitian St Thomas' Hospital, London 12:20 << LUNCH >> Afternoon Session Afternoon Chair: Dr Rosy Wells 13:00 Preventing food allergy: what to tell parents Dr Michael Perkin Consultant Allergist and Reader in Clinical Epidemiology St George's Hospital, London 13:30 Priorities to Tell Families Living With Food Allergy Professor Adam Fox Professor in Paediatric Allergy St Thomas' Hospital, London 14:00 QUICK FIRE: Adrenaline auto-injector guide IN PRACTICE: Spare pens in schools Alia Boardman Clinical Nurse Specialst in Paediatric Allergy St George's Hospital, London 14:30 << BREAK >> 15:00 Managing Long-Term Asthma in a Virtual World Dr Richard Iles Paediatric Respirology Consultant St Thomas' Hospital, London 15:40 How to recognise and treat allergic rhinitis Dr Anne Chsitopher Consultant Paediatrician and Allergist St George's Hospital, London 16:10 New inhalers and nasal sprays Katherine Knight Clinical Nurse Specialist in Paediatric Allergy St Thomas' Hospital, London 16:40 Webinar feedback and Close of meeting

|
Scooped by
Gilbert C FAURE
March 8, 2020 3:06 PM
|
Abstract Background Chronic spontaneous urticaria (CSU) is characterized by recurrent itchy weals and/or angioedema and is believed to be driven by mast cell activation. It was shown that excessive...

|
Suggested by
Société Francaise d'Immunologie
June 8, 2019 1:58 PM
|
Services on Demand Journal Article Indicators Cited by SciELO Access statistics Related links Cited by Google Similars in SciELO Similars in Google Share Anais Brasileiros de Dermatologia Print version ISSN 0365-0596On-line version ISSN 1806-4841 An. Bras. Dermatol. vol.94 no.2 supl.1 Rio de Janeiro Mar./Apr. 2019 Epub June 03, 2019 http://dx.doi.org/10.1590/abd1806-4841.2019940210 ; ATOPIC DERMATITIS Consensus on the therapeutic management of atopic dermatitis - Brazilian Society of Dermatology* 1Department of Dermatology, Faculdade de Medicina, Universidade de São Paulo, São Paulo (SP), Brazil. 2Dermatology Service, Irmandade Santa Casa de Misericórdia de Porto Alegre, Porto Alegre (RS), Brazil. 3Dermatology Service, Universidade Federal de Ciências da Saúde de Porto Alegre, Porto Alegre (RS), Brazil. 4Dermatology Service, Hospital de Clínicas de Porto Alegre, Universidade Federal do Rio Grande do Sul, Porto Alegre (RS), Brazil. 5Dermatology Service, Hospital das Clínicas, Universidade Federal de Minas Gerais, Belo Horizonte (MG), Brazil. 6Medical Dermatology Residency Program, Instituto de Ensino e Pesquisa, Hospital Sírio-Libanês, São Paulo (SP), Brazil. 7Clinic of Dermatology, Department of Medicine, Faculdade de Medicina da Santa Casa de São Paulo, São Paulo (SP), Brazil. 8Dermatology Service, Universidade do Estado do Pará, Belém (PA), Brazil. 9Dermatology Service, Hospital Municipal Jesus, Rio de Janeiro (RJ), Brazil. 10Dermatology Outpatient Clinic, Discipline of Dermatology, Faculdade de Medicina do ABC, Santo André (SP), Brazil. 11Dermatology Service, Hospital do Servidor Público Estadual, São Paulo (SP), Brazil. 12Dermatology Service, Complexo Hospitalar Padre Bento, Guarulhos (SP), Brazil. ABSTRACT: BACKGROUND: Atopic dermatitis is a highly prevalent inflammatory and pruritic dermatosis with a multifactorial etiology, which includes skin barrier defects, immune dysfunction, and microbiome alterations. Atopic dermatitis is mediated by genetic, environmental, and psychological factors and requires therapeutic management that covers all the aspects of its complex pathogenesis. OBJECTIVES: The aim of this article is to present the experience, opinions, and recommendations of Brazilian dermatology experts regarding the therapeutic management of atopic dermatitis. METHODS: Eighteen experts from 10 university hospitals with experience in atopic dermatitis were appointed by the Brazilian Society of Dermatology to organize a consensus on the therapeutic management of atopic dermatitis. The 18 experts answered an online questionnaire with 14 questions related to the treatment of atopic dermatitis. Afterwards, they analyzed the recent international guidelines on atopic dermatitis of the American Academy of Dermatology, published in 2014, and of the European Academy of Dermatology and Venereology, published in 2018. Consensus was defined as approval by at least 70% of the panel. RESULTS/CONCLUSION: The experts stated that the therapeutic management of atopic dermatitis is based on skin hydration, topical anti-inflammatory agents, avoidance of triggering factors, and educational programs. Systemic therapy, based on immunosuppressive agents, is only indicated for severe refractory disease and after failure of topical therapy. Early detection and treatment of secondary bacterial and viral infections is mandatory, and hospitalization may be needed to control atopic dermatitis flares. Novel target-oriented drugs such as immunobiologicals are invaluable therapeutic agents for atopic dermatitis. Keywords: Atopic dermatitis; Interleukins; Inflammation; Keratinocytes; Skin barrier INTRODUCTION Atopic dermatitis (AD) is a chronic inflammatory skin disease, with lesions showing typical morphology and distribution, and whose hallmark is intense pruritus. AD presents in patients with a personal or family history of atopic diseases such as asthma, rhinitis, or AD itself. It is one of the most frequent diseases of childhood, and its prevalence reaches up to 20% in infants and 2.1 to 4.9% in adults in Europe, North America, and Japan.1-3 Annual incidence of new cases of AD in patients below the age of 17 in the US is 11%; 85% of AD patients first manifest the disease before the age of 5, but 20-40% of children with AD persist with the skin disease in adulthood. 4,5 In the Brazilian population, prevalence of AD symptoms according to Solé et al. was 8.2% in children and 5.0% in adolescents. 6 Due to the complex pathogenesis of AD, which involves skin barrier defects, immune dysfunction, and microbiome alterations mediated by genetic, environmental, and psychological triggers, a single therapeutic approach is hardly capable of achieving disease control. 7 Increased transepidermal water loss (TEWL), decreased stratum corneum water content, and reduced expression of skin barrier proteins such as filaggrin and claudin 1 are the main alterations of the skin barrier in individuals with AD. 8-10 Of note is the cytokine dysregulation, leading to Th2, Th1, Th17, and Th22 polarization, which varies according to age, ethnicity, and AD phase. 11-13 Skin microbiome plays a crucial role in AD; about 90% of the skin of atopic individuals is colonized by Staphylococcus aureus (S. aureus). 14 The diversity of skin microbiome of AD patients shows temporal shifts, with a predominance of S. aureus during flares and Streptococcus, Propionibacterium, and Corynebacterium after treatment. 15 AD remains a challenging disease. Ideal treatment is targeted to long-term disease control with reduction of flares and maintenance of good quality of life. Moreover, treatment approaches depend on geographic, economic, and genotypic/phenotypic variations. This paper aims to communicate the experience, opinions, and recommendations of Brazilian dermatology experts on atopic dermatitis treatment. METHODS Eighteen faculty members from 10 university hospitals with expertise in AD were appointed by the Brazilian Society of Dermatology. The first step was the application of an online questionnaire with 14 questions regarding the management of AD patients by the experts at university hospitals. Table 1 shows the compiled answers. Table 1 answers/total Number of AD patients seen per month <50 9/18 >50 9/18 Number of patients seen in public institutions < 50 2 / 18 >50 16 / 18 Number of patients seen in private practice < 50 4 / 18 >50 13 / 18 Treatment based on published guidelines (12/18) American 5 / 12 European 5 / 12 Other 2 / 12 Topical therapy: Topical corticosteroids 17 / 18 Calcineurin inhibitors 16 / 18 Topical antibiotics 6 / 18 Other-moisturizers 7 / 18 Systemic therapy: Cyclosporine 14 / 18 Azathioprine 3 / 18 Methotrexate 11 / 18 Mycophenolatemofetil 1 / 18 Oral steroids 5 / 18 Immunobiologicaltherapy 0 / 18 Phototherapy (narrow-band UVB) Yes 15 / 18 No 3 / 18 Use of antimicrobials during flares Topical 10 / 18 Systemic 16 / 18 The second step was the analysis of recent international guidelines (American Academy of Dermatology, published in 2014, and the European Academy of Dermatology and Venereology, published in 2018). 16-21 All sections and recommendations regarding AD treatment were discussed with the 18 experts, and consensus was defined as approval by at least 70% of the panel. This paper expresses their opinions regarding international guidelines for AD treatment and provides practical guidance for dermatologists in Brazil. RESULTS/DISCUSSION Table 1 shows the data obtained from the applied questionnaire. The majority of experts (17/18) who answered the questionnaire work in public and private institutions. About 50% of the specialists see more than 50 AD patients/month, mostly at public hospitals. Twelve out of 18 of the dermatologists follow published consensuses, with emphasis on the American and European guidelines. The most widely used topical treatments are corticosteroids, followed by calcineurin inhibitors. The first choice for systemic therapy was cyclosporin, followed by methotrexate and azathioprine. Baseline therapy and preventive measures The recent guidelines are in accordance regarding baseline therapy. Key steps include maintenance of the skin barrier through the constant use of emollients, which recover the function of the damaged skin barrier in AD and consequently protect the skin from allergen penetration and subsequent inflammation. 22 Skin hydration improves xerosis and reduces pruritus, sparing topical corticosteroid use. Cleansing eliminates crusts and reduces bacterial contamination. The use of substances with physiological pH is recommended, and baths should last no longer than five minutes. 23 Sodium hypochlorite baths (bleach) may not always change the severity of AD but appear to reduce the need for topical anti-inflammatory drugs and antibiotics. 24 Daily bathing is possible for regular skin hydration, and emollients should be applied on slightly wet skin, immediately after drying; application twice daily is usually sufficient. 25 Some emollients have additional ingredients such as urea and propylene glycol, which may lead to skin irritation, and there is still inconclusive evidence about superiority of emollients enhanced with components of the skin barrier such as ceramides. 25,26 Recent concepts regarding the microbiome and the skin highlight that the cutaneous microbiome in AD is not as heterogeneous as in healthy individuals, with the predominance of Staphylococcus aureus (S. aureus). 15,27 Recovery of the skin barrier by adjusting the inflammatory response reestablishes the skin microbiome in AD patients. 28,29 Bacterial lysates or topical application of commensal bacteria are promising, but skin hydration itself is able to recover the skin microbiome. 15,30 There is evidence for early use of emollients in atopic dermatitis-prone children (three months of age and older) in the prevention of AD. 28,29 Recommendations by the dermatology experts for baseline therapy: Daily cleansing for up to 5 minutes with mild agents with adequate pH Emollient application twice daily on slightly wet skin is the main component of baseline therapy Aeroallergens Aeroallergens are relevant triggering factors of AD flares.31-33 Exacerbation of an eczematous lesion after skin contact or inhalation has been reported, but studies are still inconclusive. 32 The skin prick test and specific IgE are routinely utilized but have a low positive predictive value. 32 In the present panel of dermatology experts, 89% do not perform the skin prick test or RAST as part of routine practice. Food allergy One-third of the children with moderate/severe AD have associated food allergy; however, food allergy is not the cause of AD. 34 Restrictive diets should only be prescribed for children with proven food allergy. 34,35 The published guidelines recommend restrictive diets only for those patients with a positive oral challenge test, the gold standard assay for food allergy. 19,34-36 The detection of specific IgE to food through prick or serological tests does not prove food allergy, and their positive predictive value is low. 37 The present panel of dermatology experts does not recommend restrictive diets, but considers that food allergy may be investigated in children with severe, treatment-resistant AD and in those with a history of flares following ingestion of specific foods. Contact dermatitis Contact dermatitis is present in 40-65% of AD patients, usually exacerbating the existing eczema. 38 The patch test is recommended for refractory AD with atypical skin lesions. 39 Patients should be tested for fragrances, preservatives, topical corticosteroids, and other topical components. 38 Patients are more prone to develop occupational dermatoses, since AD exacerbates the irritant effect of allergens in certain professions such as hairdressers, mechanics, metalworkers, janitorial workers, and nurses, in whom hand eczema is commonly reported. 40 Preventive measures should be taken in order to reduce the incidence of AD in such patients. Fifty percent of the expert group recommend patch tests. The main problem is the difficulty in performing the test, since ideal sites are usually limited in AD patients. Topical anti-inflammatory therapy Topical anti-inflammatory therapy is the mainstay of AD treatment. Anti-inflammatory agents must have sufficient potency and should be applied on the skin lesions according to the recommendations and not exceeding the allowed amount per day. 41 Topical corticosteroids (TC) TC are the first line treatment for AD, with strong evidence of their superiority over placebo. 42 They are classified according to their potency based on vasoconstrictive effects, and every clinician should be aware of their potential local and systemic adverse effects, such as cutaneous atrophy and adrenal suppression.43,44 Strategies defining the use of TC vary according to their potency, but the suggested applied amounts of topical corticosteroids follow the fingertip unit rule.19,20 In the European guidelines, the approximate total amount of TC per month is 15g in infants, 30g in children, and 60-90g in adolescents and adults.20 The choice of corticosteroid and its vehicle depend on the affected site, the patients’ age and the severity and clinical phase of AD. Wet-wrap dressings may improve AD flares, and ultrahigh potent topical corticosteroids should be applied for up to two weeks.29,45 TC use depends on the vehicle; as a cream, they should be applied 15 minutes before the moisturizer, and as an ointment, applied 15 minutes prior to the moisturizer.41 Corticosteroid phobia is a relevant matter that should be addressed, especially due to its influence on adherence to treatment; it varies according to the country and culture.46 Topical corticosteroids are the first-line topical treatment for AD, according to the experts. Calcineurin inhibitors (topical immunomodulators or TIM) Tacrolimus and pimecrolimus are second-line non-corticosteroid, anti-inflammatory therapies for AD with proven efficacy.20,47 The most widely reported adverse effect is burning sensation during the initial days of use (especially with tacrolimus); they do not induce skin atrophy, which makes them useful for application on eyelids, perioral lesions, axillae, and genitals.48 Despite a black box warning in the package insert, studies have not reported an increased risk of lymphoma with the topical use of TIM at therapeutic doses.49 Intermittent use of TIM is recommended above two years of age.20 Eighty percent of the Brazilian experts use TIM as a second-line therapy for AD. Proactive treatment Proactive treatment has been proposed in published guidelines. It consists of long-term use of topical anti-inflammatory agents, either TC or TIM (tacrolimus), twice a week in previously affected areas, combined with moisturizers. 29,45,50,51 The rationale for proactive treatment is based on its efficacy and long-term safety (up to one year), reducing the number of flares and improving the quality of life of atopic patients. 50,51 The Brazilian experts recommend proactive treatment with TC or TIM in AD patients. Topical antimicrobial therapy Colonization by S. aureus is frequent on the skin of AD patients and is much higher than in non-atopic individuals (100% vs. 30%). 52-54Fortunately, the skin and nares of AD patients are not frequently colonized by methicillin-resistant S. aureus (MRSA) (7.4 and 4%, respectively). 54 The American Academy of Dermatology does not recommend the use of topical antibiotics, since they do not show clear benefits for AD patients. However, the use of 0.005% sodium chlorine in bathwater may be helpful in children and is recommended by the EADV. 17,20 During flares, 100% of the Brazilian experts use antibiotics. About 1/3 of the experts use topical antibiotics in acute phases of AD for short periods (up to one week). Recommendations for topical therapy in AD: TC are the first-line treatment for AD patients and must be carefully prescribed according to their potency and vehicle. Patient’s age, site, and phase of AD lesions are key factors when choosing TC. TIM constitute the second-line treatment for AD and are suitable for application on areas with high risk of corticosteroid-induced atrophy. Proactive therapy with either TC or TIM is safe, reduces flares and AD severity, and is indicated as long-term maintenance therapy. The use of topical antibiotics and antiseptics is still variable. Topical antibiotics can be used for short periods, and bleachers (0.005% sodium hypochlorite may be useful for pediatric AD). Wet-wrap bandages or occlusive treatment during hospitalization are helpful measures for improving flares. In patients that fail to respond to topical treatment, the following should be considered: -differential diagnoses of AD -lack of adherence -contact dermatitis -secondary infection (bacterial, viral, or fungal) -indication for systemic therapy Systemic treatment Systemic treatment of AD is recommended in moderate to severe cases that fail to respond topical therapies. Before initiating systemic treatment, it is mandatory to avoid aggravating factors, to diagnose and treat secondary infections, and to rule out differential diagnoses. The option for systemic therapy should also include the impact of the disease on the patient’s quality of life and a careful balance of risks and benefits with the chosen medication. 55,56 Phototherapy Phototherapy is a valid adjuvant therapeutic option, especially for chronic AD and in adults. It improves pruritus, thus reducing insomnia. 57 Ultraviolet B (UVB), narrow-band UVB, and psoralen + UVA (PUVA) are the main modalities. 12,57 UVB-NB (311-313nm) is the most widely used form and can be indicated for children. UVA1 (340-400nm) is seldom used in Brazil but is useful for flares. 21,57 In the Brazilian consensus group, 83% recommend this treatment modality, especially for the chronic phase of AD. Phototherapy improves clinical signs and reduces pruritus and bacterial colonization, thus being a steroid-sparing measure. It is important to avoid this treatment in patients with recurrent herpes simplex infection or history of eczema herpeticum. A limiting factor for this therapeutic modality is lack of adherence to long-term treatment. Antihistamines Oral antihistamines that block the histamine 1 receptor (H1R) have been prescribed for AD patients for decades; however, there are few randomized studies that evaluate their real efficacy in AD. 21 The aim of systemic antihistamines in AD is to allow better quality of sleep, since their role as anti-inflammatory agents in AD is controversial. There is no evidence of improvement of severity scores in randomized studies, and first-generation drugs are prescribed due to their sedative effect and to the relief of other conditions related to AD, such as asthma, rhinoconjunctivitis, dermographism, and urticaria. 21,58 However, our group stresses that the quality of sleep induced by anti-H1R drugs is not ideal, since they do not alter the REM phase. 21,58 Our group recommends the use of first-generation antihistamines (hydroxyzine and chlorpheniramine) based only on their sedative effect. Anti-inflammatory agents Cyclosporin A (CyA) Cyclosporin A is approved in many European countries and in Brazil for severe AD. The U.S. FDA approves it for psoriasis. The initial dose for children and adults varies from 3 to 5 mg/kg/day, and the maintenance dose is from 2.5 to 3 mg/kg/day. 55,59-61 Clinical improvement can be observed after 2-8 weeks; CyA is recommended for up to 2 years with constant monitoring of blood pressure and kidney function. 55,59-61 Periodic intervals of 3-6 months off therapy decreases the occurrence of side effects. 62 The average length of treatment with CyA is 3-12 months, and the drug is usually considered first-line treatment for treatment-resistant AD and in acute flares. 63 Pregnancy is not a contraindication to CyA use. 63 Although CyA leads to prompt improvement in severity scores after 2 weeks from the initial dose, reactivation of AD after the drug’s suspension is equally rapid, occurring in 2 weeks. 63 Methotrexate (MTX) MTX can be indicated as initial treatment for moderate/severe AD, recalcitrant to topical treatment with corticosteroids. The drug has a good safety profile and is indicated for long-term maintenance; clinical efficacy is reached after 8-12 weeks of administration.21,61,63 The therapeutic dose varies from 15 to 25mg/week for adults and 10-15mg/m2/week for children (oral, intravenous, or subcutaneous), and folate should be added to the treatment, usually 1-2 days after MTX. 21,61,63 Average length of treatment ranges from 6 to 12 months, and clinical improvement is seen at 8-12 weeks from the initial dose. Side effects include hematological disorders, liver enzyme alterations, and gastrointestinal discomfort. Its use is recommended for up to 2 years, with constant monitoring of bone marrow and liver function. 21,61,63,64 Contraception is mandatory, since the drug is considered category X. 61,63 Azathioprine (AZA) AZA can be indicated as systemic treatment for refractory AD. Peak efficacy of AZA is reached after 8-12 weeks of use. 63 The initial dose is usually 50 mg/day for 1-2 weeks, increased thereafter to 2-3 mg/kg/day. 63,65 It can increase the risk of non-melanoma skin cancer and lymphoma. 66,67 Thiopurine methyltransferase enzyme (TPMT) levels should be measured whenever possible, since TPMT deficiency while in use of AZA can lead to bone marrow aplasia. 65 It can be prescribed for children (off label for AD) and is subject to restricted indication during pregnancy. 63,68 Mycophenolate mofetil (MMF) Clinical efficacy of MMF is reached after 8-12 weeks of use (off label in AD), and the drug has a good safety profile. 21,63 The recommended doses in adults are 1-2g/day (starting dose) and 2-3g/ day (maintenance); the pediatric doses are 20-50mg/kg/day (starting dose) and 30-50mg/kg/day (maintenance).21,68 Gastrointestinal and hematological side effects have been reported. 21,63 Systemic corticosteroids (SC) There are few randomized controlled studies regarding the use of systemic corticosteroids in AD. In the 2018 European consensus, SC are used in exceptional cases of AD, but only for one week.21 There is a rapid clear up of skin lesions , but severe rebound tends to occur in 2 weeks. 21 One controlled trial indicates lower efficacy of systemic prednisolone in comparison to CyA in severe AD.21,69 Position/recommendations for the use of systemic anti-inflammatory drugs in AD: CyA and MTX are the most widely used systemic drugs for severe refractory AD. CyA leads to fast improvement of AD severity scores after 2 weeks of initial treatment, but reactivation of AD after drug suspension is equally fast, occurring in 2 weeks. MTX can be used as the initial systemic medication for refractory moderate/severe AD and is indicated for long-term maintenance. Clinical efficacy is reached after 8-12 weeks of administration. Oral corticosteroids are used in exceptional cases for short periods (up to 1 week). Few dermatologists have experience with mycophenolate mofetil or azathioprine. Treatment of secondary infections (bacterial, viral, or fungal) S. aureus and Streptococcus pyogenes are the most common bacterial agents in AD. They are detected in more than 90% of AD lesions.53 Systemic antibiotics are reserved for patients with clinical evidence of infection, and cephalosporins are the first choice of treatment.21,70 Extensive viral infections such as eczema herpeticum (EH) are seen in AD patients. Skin barrier defects, including mutations of the filaggrin and claudin1 genes or abnormalities in IFN-gamma response may increase the risk of EH. 12,71 Risk factors for EH include early severe AD, high IgE levels, eosinophilia, and associated food allergy and asthma.14,45,63,72 Treatment for localized EH is oral acyclovir. Systemic involvement with fever, lethargy, headache, nausea, and dizziness requires hospitalization and intravenous acyclovir. 72 As for fungal infections in patients with AD, Malassezia spp. appears to contribute to skin inflammation during flares, and there is an anti-IgE response to immunogenic proteins released by some Malassezia species. 73 Recommendations by the Brazilian experts: oral antibiotics are indicated when there are signs of bacterial superinfection of the skin; cephalosporins are the first choice, followed by sulfamethoxazole-trimethoprim. Eczema herpeticum must be treated with systemic antiviral drugs; when it is followed by systemic symptoms and signs, hospitalization and intravenous antiviral therapy are indicated. AD patients with head and neck involvement may benefit from treatment with antifungal agents. Education and AD AD has a strong impact on the quality of life of patients and caregivers due to its chronic course and intense pruritus. 21,45,74 Sleep loss, school and work absenteeism, social isolation, depression, and suicidal ideation may be present. 56,74 Low treatment adherence is common in AD, and educational programs are needed to reinforce the patient’s understanding of the disease complexity and therapeutic approaches. 75 Various models focusing on AD education and with multidisciplinary approaches have shown subjective and objective improvement of AD worldwide. 75-79 Future perspectives Immunobiologicals and small molecules are targeted therapies that have been developed for many inflammatory, autoimmune, and oncologic diseases. Crisaborole ointment is a topical phosphodiesterase 4 (PDE4) inhibitor that was approved in the USA in 2017 for patients above the age of 2 years with mild/moderate AD. 80 Dupilumab is a human monoclonal antibody for AD that blocks the alfa-chain receptor for IL-4 and IL-13 (dupilumab) and is approved for adults with moderate/severe AD. 81 Its efficacy after 16 weeks as monotherapy (initial dose: 600 mg, followed by 300 mg every 2 weeks, SC), measured by the reduction of eczema severity scores (EASI) was 82.5% (EASI 50), 60.3% (EASI 75), and 36.5% (EASI 90).81-83 Improvement of skin lesions and reduction of pruritus improved 2 weeks after initiating treatment. 81-83The studies show sustained long-term efficacy (one year) with dupilumab combined with TC in AD patients.84,85 The main adverse event reported with dupilumab was conjunctivitis, detected in 25-50% of AD patients.85,86 There are ongoing studies (phases 2-3) with novel immunobiologicals and small molecules for AD treatment. See Chart 1. 87,88 Chart 1 Agent Target Administration Phase Tralokinumab IL-13 SC 3 Lebrikizumab IL-13 SC 2 Nemolizumab IL-31Ra SC 3 Apremilast PDE4 PO 2 ILV-094 IL-22 IV terminated Secukinumab IL-17 SC 2 Baricitinib JAK1/2 PO 2 Upadacitinib JAK1 SC 2 ZPL389 H4R PO 2 Tezepelumab TSLP SC 2 Serlopitant NKR1 PO 2 IL=interleukin; R=receptor PDE=phosphodiesterase; JAK= janus kinase; H=histamine; TSLP=thymic stromal lymphopoietin; NKR=neurokinin receptor; SC= subcutaneously; PO= per oral; IV= intravenously Source: Wang, et al, 201687 and Lee, et al, 2018.88 Chart 1: New systemic drugs for AD treatment. 87,88 CONCLUSIONS Despite the cultural and economic differences between Brazil, USA, and Europe, including in access to immunobiological therapies, the ideal management of AD is based on a better understanding of disease pathogenesis and knowledge of treatment strategies. Basic treatment for AD includes skin hydration, topical anti-inflammatory therapy, avoidance of aggravating factors, and educational programs with a multidisciplinary approach. Systemic therapy should be only indicated for refractory or severe disease after attempts with topical therapy. Secondary infections must be diagnosed early and treated promptly, and hospitalization may be necessary to control flares (Figure 1). Figure 1 Novel target-oriented drugs are invaluable tools for AD treatment. *Work conducted at the Sociedade Brasileira de Dermatologia, Rio de Janeiro (RJ), Brazil. Financial support: None. REFERENCES 1 Eichenfield LF, Ellis CN, Mancini AJ, Paller AS, Simpson EL. Atopic dermatitis: epidemiology and pathogenesis update. Semin Cutan Med Surg. 2012;31(3 Suppl):S3-5. [ Links ] 2 Watson W, Kapur S. Atopic dermatitis. Allergy Asthma Clin Immunol. 2011;7( Suppl 1):S4 [ Links ] 3 Barbarot S, Auziere S, Gadkari A, Girolomoni G, Puig L, Simpson EL, et al. Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy. 2018;73:1284-1293 [ Links ] 4 Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112(6 Suppl):S118-27. [ Links ] 5 Ozkaya E. Adult-onset atopic dermatitis. J Am Acad Dermatol. 2005;52:579-82. [ Links ] 6 S Solé D, Camelo-Nunes IC, Wandalsen GF, Mallozi MC, Naspitz CK; Brazilian ISAAC Group. Prevalence of atopic eczema and related symptoms in Brazilian schoolchildren: results from the International Study of Asthma and Allergies in Childhood (ISAAC) phase 3. J Investig Allergol Clin Immunol. 2006;16:367-76. [ Links ] 7 Orfali RL, Zaniboni MC, Aoki V. Profile of skin barrier proteins and cytokines in adults with atopic dermatitis. G Ital Dermatol Venereol. 2017;152:140-7. [ Links ] 8 Batista DI, Perez L, Orfali RL, Zaniboni MC, Samorano LP, Pereira NV, et al. Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J Eur Acad Dermatol Venereol. 2015;29:1091-5. [ Links ] 9 Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008;121:1337-43. [ Links ] 10 Gittler JK, Shemer A, Suárez-Fariñas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130:1344-54. [ Links ] 11 Czarnowicki T, Gonzalez J, Shemer A, Malajian D, Xu H, Zheng X et al. Severe atopic dermatitis is characterized by selective expansion of circulating TH2/ TC2 and TH22/TC22, but not TH17/TC17, cells within the skin-homing T-cell population. J Allergy Clin Immunol. 2015;136:104-115.e7. [ Links ] 12 Leung DY. New insights into atopic dermatitis: role of skin barrier and immune dysregulation. Allergol Int. 2013;62:151-61. [ Links ] 13 Shi B, Bangayan NJ, Curd E, Taylor PA, Gallo RL, Leung DYM, et al. The skin microbiome is different in pediatric versus adult atopic dermatitis. J Allergy Clin Immunol. 2016;138:1233-6. [ Links ] 14 Ong PY, Leung DY. Bacterial and Viral Infections in Atopic Dermatitis: a Comprehensive Review. Clin Rev Allergy Immunol. 2016;51:329-37. [ Links ] 15 Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850-9. [ Links ] 16 Eichenfield LF, Tom WL, Chamlin SL, Feldman SR, Hanifin JM, Simpson EL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-51. [ Links ] 17 Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-32. [ Links ] 18 Sidbury R, Davis DM, Cohen DE, Cordoro KM, Berger TG, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-49 [ Links ] 19 Sidbury R, Tom WL, Bergman JN, Cooper KD, Silverman RA, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: Section 4. Prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014;71:1218-33. [ Links ] 20 Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657-82. [ Links ] 21 Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32:850-78. [ Links ] 22 Åkerström U, Reitamo S, Langeland T, Berg M, Rustad L, Korhonen L, et al. Comparison of Moisturizing Creams for the Prevention of Atopic Dermatitis Relapse: A Randomized Double-blind Controlled Multicentre Clinical Trial. Acta Derm Venereol. 2015;95:587-92. [ Links ] 23 Blume-Peytavi U, Cork MJ, Faergemann J, Szczapa J, Vanaclocha F, Gelmetti C. Bathing and cleansing in newborns from day 1 to first year of life: recommendations from a European round table meeting. J Eur Acad Dermatol Venereol. 2009;23:751-9. [ Links ] 24 Hon KL, Tsang YC, Lee VW, Pong NH, Ha G, Lee ST, et al. Efficacy of sodium hypochlorite (bleach) baths to reduce Staphylococcus aureus colonization in childhood onset moderate-to-severe eczema: A randomized, placebo-controlled cross-over trial. J Dermatolog Treat. 2016;27:156-62. [ Links ] 25 Koutroulis I, Pyle T, Kopylov D, Little A, Gaughan J, Kratimenos P. The Association Between Bathing Habits and Severity of Atopic Dermatitis in Children. Clin Pediatr (Phila). 2016;55:176-81. [ Links ] 26 Blume-Peytavi U, Lavender T, Jenerowicz D, Ryumina I, Stalder JF, Torrelo A, et al. Recommendations from a European Roundtable Meeting on Best Practice Healthy Infant Skin Care. Pediatr Dermatol. 2016;33:311-21. [ Links ] 27 Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190-2. [ Links ] 28 Simpson EL, Chalmers JR, Hanifin JM, Thomas KS, Cork MJ, McLean WH, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-23. [ Links ] 29 Mohan GC, Lio PA. Comparison of Dermatology and Allergy Guidelines for Atopic Dermatitis Management. JAMA Dermatol. 2015;151:1009-13. [ Links ] 30 Myles IA, Earland NJ, Anderson ED, Moore IN, Kieh MD, Williams KW, et al. First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight. 2018;3. pii: 120608 [ Links ] 31 Werfel T, Heratizadeh A, Niebuhr M, Kapp A, Roesner LM, Karch A, et al. Exacerbation of atopic dermatitis on grass pollen exposure in an environmental challenge chamber. J Allergy Clin Immunol. 2015;136:96-103.e9. [ Links ] 32 Garritsen FM, ter Haar NM, Spuls PI. House dust mite reduction in the management of atopic dermatitis. A critically appraised topic. Br J Dermatol. 2013;168:688-91 [ Links ] 33 Lorenzini D, Pires M, Aoki V, Takaoka R, Souza RL, Vasconcellos C. Atopy patch test with Aleuroglyphus ovatus antigen in patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2015;29:38-41. [ Links ] 34 Eigenmann PA, Sicherer SH, Borkowski TA, Cohen BA, Sampson HA. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998;101:E8. [ Links ] 35 Bath-Hextall F, Delamere FM, Williams HC. Dietary exclusions for improving established atopic eczema in adults and children: systematic review. Allergy. 2009;64:258-64. [ Links ] 36 Thompson MM, Tofte SJ, Simpson EL, Hanifin JM. Patterns of care and referral in children with atopic dermatitis and concern for food allergy. Dermatol Ther. 2006;19:91-6. [ Links ] 37 Cuomo B, Indirli GC, Bianchi A, Arasi S, Caimmi D, Dondi A, et al. Specific IgE and skin prick tests to diagnose allergy to fresh and baked cow's milk according to age: a systematic review. Ital J Pediatr. 2017;43:93. [ Links ] 38 Herro EM, Matiz C, Sullivan K, Hamann C, Jacob SE. Frequency of contact allergens in pediatric patients with atopic dermatitis. J Clin Aesthet Dermatol. 2011;4:39-41. [ Links ] 39 Arkwright PD, Motala C, Subramanian H, Spergel J, Schneider LC, Wollenberg A, et al. Management of difficult-to-treat atopic dermatitis. J Allergy Clin Immunol Pract. 2013;1:142-51. [ Links ] 40 Borok J, Matiz C, Goldenberg A, Jacob SE. Contact Dermatitis in Atopic Dermatitis Children-Past, Present, and Future. Clin Rev Allergy Immunol. 2018. [Epub ahead of print] [ Links ] 41 Ring J, Alomar A, Bieber T, Deleuran M, Fink-Wagner A, Gelmetti C, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) part I. J Eur Acad Dermatol Venereol. 2012;26:1045-60 [ Links ] 42 Van Der Meer JB, Glazenburg EJ, Mulder PG, Eggink HF, Coenraads PJ. The management of moderate to severe atopic dermatitis in adults with topical fluticasone propionate. The Netherlands Adult Atopic Dermatitis Study Group. Br J Dermatol. 1999;140:1114-21. [ Links ] 43 Luger TA. Balancing efficacy and safety in the management of atopic dermatitis: the role of methylprednisolone aceponate. J Eur Acad Dermatol Venereol. 2011;25:251-8. [ Links ] 44 Walsh P, Aeling JL, Huff L, Weston WL. Hypothalamus-pituitary-adrenal axis suppression by superpotent topical steroids. J Am Acad Dermatol. 1993;29:501-3. [ Links ] 45 Lebwohl MG, Del Rosso JQ, Abramovits W, Berman B, Cohen DE, Guttman E, et al. Pathways to managing atopic dermatitis: consensus from the experts. J Clin Aesthet Dermatol. 2013;6(7 Suppl):S2-S18. [ Links ] 46 Stalder JF, Aubert H, Anthoine E, Futamura M, Marcoux D, Morren MA et al. Topical corticosteroid phobia in atopic dermatitis: International feasibility study of the TOPICOP score. Allergy. 2017;72:1713-9. [ Links ] 47 Alomar A, Berth-Jones J, Bos JD, Giannetti A, Reitamo S, Ruzicka T, et al. The role of topical calcineurin inhibitors in atopic dermatitis. Br J Dermatol. 2004;151(Suppl 70):S3-27. [ Links ] 48 Reitamo S, Rissanen J, Remitz A, Granlund H, Erkko P, Elg P, et al. Tacrolimus ointment does not affect collagen synthesis: results of a single-center randomized trial. J Invest Dermatol. 1998;111:396-8. [ Links ] 49 Arellano FM, Wentworth CE, Arana A, Fernández C, Paul CF.. Risk of lymphoma following exposure to calcineurin inhibitors and topical steroids in patients with atopic dermatitis. J Invest Dermatol. 2007;127:808-16. [ Links ] 50 Thaçi D, Reitamo S, Gonzalez Ensenat MA, Moss C, Boccaletti V, Cainelli T, et al. Proactive disease management with 0.03% tacrolimus ointment for children with atopic dermatitis: results of a randomized, multicentre, comparative study. Br J Dermatol. 2008;159:1348-56. [ Links ] 51 Wollenberg A, Reitamo S, Girolomoni G, Lahfa M, Ruzicka T, Healy E, et al. Proactive treatment of atopic dermatitis in adults with 0.1% tacrolimus ointment. Allergy. 2008;63:742-50. [ Links ] 52 Orfali RL, Rivitti E, Sato MN, Takaoka R, Aoki V. Adult atopic dermatitis: Evaluation of TH17 and TH22 cytokines in peripheral blood mononuclear cells induced by staphylococcal enterotoxins A and B. J Am Acad Dermatol. 2012;66:AB68-AB. [ Links ] 53 Breuer K, Wittmann M, Kempe K, Kapp A, Mai U, Dittrich-Breiholz O, et al. Alphatoxin is produced by skin colonizing Staphylococcus aureus and induces a T helper type 1 response in atopic dermatitis. Clin Exp Allergy. 2005;35:1088-95. [ Links ] 54 Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics. 2009;123:e808-14. [ Links ] 55 Simpson EL, Bruin-Weller M, Flohr C, Ardern-Jones MR, Barbarot S, Deleuran M, et al. When does atopic dermatitis warrant systemic therapy? Recommendations from an expert panel of the International Eczema Council. J Am Acad Dermatol. 2017;77:623-633. [ Links ] 56 Ring J, Alomar A, Bieber T, Deleuran M, Fink-Wagner A, Gelmetti C, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) Part II. J J Eur Acad Dermatol Venereol. 2012;26:1176-93 [ Links ] 57 Garritsen FM, Brouwer MW, Limpens J, Spuls PI. Photo(chemo)therapy in the management of atopic dermatitis: an updated systematic review with implications for practice and research. Br J Dermatol. 2014;170:501-13. [ Links ] 58 Diepgen TL; Early Treatment of the Atopic Child Study Group. Long-term treatment with cetirizine of infants with atopic dermatitis: a multi-country, double-blind, randomized, placebo-controlled trial (the ETAC trial) over 18 months. Pediatr Allergy Immunol. 2002;13:278-86. [ Links ] 59 Brasch J, Becker D, Aberer W, Bircher A, Kränke B, Jung K, et al. Guideline contact dermatitis: S1-Guidelines of the German Contact Allergy Group (DKG) of the German Dermatology Society (DDG), the Information Network of Dermatological Clinics (IVDK), the German Society for Allergology and Clinical Immunology (DGAKI), the Working Group for Occupational and Environmental Dermatology (ABD) of the DDG, the Medical Association of German Allergologists (AeDA), the Professional Association of German Dermatologists (BVDD) and the DDG. Allergo J Int. 2014;23:126-38. [ Links ] 60 van der Schaft J, Politiek K, van den Reek JM, Christoffers WA, Kievit W, de Jong EM, et al. Drug survival for ciclosporin A in a long-term daily practice cohort of adult patients with atopic dermatitis. Br J Dermatol. 2015;172:1621-7. [ Links ] 61 Goujon C, Viguier M, Staumont-Sallé D, Bernier C, Guillet G, Lahfa M, et al. Methotrexate Versus Cyclosporine in Adults with Moderate-to-Severe Atopic Dermatitis: A Phase III Randomized Noninferiority Trial. J Allergy Clin Immunol Pract. 2018;6:562-569.e3. [ Links ] 62 van der Schaft J, van Zuilen AD, Deinum J, Bruijnzeel-Koomen CA, de Bruin-Weller MS. Serum creatinine levels during and after long-term treatment with cyclosporine A in patients with severe atopic dermatitis. Acta Derm Venereol. 2015;95:963-7. [ Links ] 63 Wollenberg A, Oranje A, Deleuran M, Simon D, Szalai Z, Kunz B, et al. ETFAD/ EADV Eczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients. J Eur Acad Dermatol Venereol. 2016;30:729-47. [ Links ] 64 Schneider L, Tilles S, Lio P, Boguniewicz M, Beck L, LeBovidge J, et al. Atopic dermatitis: a practice parameter update 2012. J Allergy Clin Immunol. 2013;131:295-9.e1-27. [ Links ] 65 Meggitt SJ, Gray JC, Reynolds NJ. Azathioprine dosed by thiopurine methyltransferase activity for moderate-to-severe atopic eczema: a double-blind, randomised controlled trial. Lancet. 2006;367:839-46. [ Links ] 66 Khan N, Abbas AM, Lichtenstein GR, Loftus EV Jr, Bazzano LA. Risk of lymphoma in patients with ulcerative colitis treated with thiopurines: a nationwide retrospective cohort study. Gastroenterology. 2013;145:1007-1015.e3. [ Links ] 67 Peyrin-Biroulet L, Khosrotehrani K, Carrat F, Bouvier AM, Chevaux JB, Simon T, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141:1621-28.e1-5. [ Links ] 68 Waxweiler WT, Agans R, Morrell DS. Systemic treatment of pediatric atopic dermatitis with azathioprine and mycophenolate mofetil. Pediatr Dermatol. 2011;28:689-94 [ Links ] 69 Schmitt J, Schäkel K, Fölster-Holst R, Bauer A, Oertel R, Augustin M, et al. Prednisolone vs. ciclosporin for severe adult eczema. An investigator-initiated double-blind placebo-controlled multicentre trial. Br J Dermatol. 2010;162:661-8. [ Links ] 70 Park HY, Kim CR, Huh IS, Jung MY, Seo EY, Park JH, et al. Staphylococcus aureus Colonization in Acute and Chronic Skin Lesions of Patients with Atopic Dermatitis. Ann Dermatol. 2013;25:410-6. [ Links ] 71 Leung DY, Gao PS, Grigoryev DN, Rafaels NM, Streib JE, Howell MD, et al. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-gamma response. J Allergy Clin Immunol. 2011;127:965-73.e1-5. [ Links ] 72 Sun D, Ong PY. Infectious Complications in Atopic Dermatitis. Immunol Allergy Clin North Am. 2017;37:75-93. [ Links ] 73 Glatz M, Bosshard P, Schmid-Grendelmeier P. The Role of Fungi in Atopic Dermatitis. Immunol Allergy Clin North Am. 2017;37:63-74. [ Links ] 74 Gieler U, Köhnlein B, Schauer U, Freiling G, Stangier U. Counseling of parents with children with atopic dermatitis. Hautarzt. 1992;43(Suppl 11):S37-42. [ Links ] 75 Staab D, von Rueden U, Kehrt R, Erhart M, Wenninger K, Kamtsiuris P, et al. Evaluation of a parental training program for the management of childhood atopic dermatitis. Pediatr Allergy Immunol. 2002;13:84-90. [ Links ] 76 Mancini AJ, Paller AS, Simpson EL, Ellis CN, Eichenfield LF. Improving the patient-clinician and parent-clinician partnership in atopic dermatitis management. Semin Cutan Med Surg. 2012;31(3 Suppl):S23-8 [ Links ] 77 Kupfer J, Gieler U, Diepgen TL, Fartasch M, Lob-Corzilius T, Ring J, et al. Structured education program improves the coping with atopic dermatitis in children and their parents-a multicenter, randomized controlled trial. J Psychosom Res. 2010;68:353-8. [ Links ] 78 Takaoka R, Aoki V. Education of Patients with Atopic Dermatitis and Their Caregivers. Pediatric Allergy Immunology and Pulmonology. 2016;29:160-3. [ Links ] 79 Weber MB, Lorenzini D, Reinehr CP, Lovato B. Assessment of the quality of life of pediatric patients at a center of excellence in dermatology in southern Brazil. An Bras Dermatol. 2012;87:697-702. [ Links ] 80 Paller AS, Tom WL, Lebwohl MG, Blumenthal RL, Boguniewicz M, Call RS, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6. [ Links ] 81 Simpson EL, Gadkari A, Worm M, Soong W, Blauvelt A, Eckert L, et al. Dupilumab therapy provides clinically meaningful improvement in patient-reported outcomes (PROs): A phase IIb, randomized, placebo-controlled, clinical trial in adult patients with moderate to severe atopic dermatitis (AD). J J Am Acad Dermatol. 2016;75:506-15. [ Links ] 82 Thaçi D, Simpson EL, Beck LA, Bieber T, Blauvelt A, Papp K, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387:40-52. [ Links ] 83 Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N Engl J Med. 2016;375:2335-48. [ Links ] 84 Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139(4 Suppl):S65-76. [ Links ] 85 Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, Weisman J, Pariser D, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303. [ Links ] 86 Wollenberg A, Ariens L, Thurau S, van Luijk C, Seegräber M, de Bruin-Weller M. Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab-clinical characteristics and treatment. J Allergy Clin Immunol Pract. 2018;6:1778-80.e1 [ Links ] 87 Wang D, Beck LA. Immunologic Targets in Atopic Dermatitis and Emerging Therapies: An Update. Am J Clin Dermatol. 2016;17:425-43. [ Links ] 88 Lee DE, Clark AK, Tran KA, Shi VY. New and emerging targeted systemic therapies: a new era for atopic dermatitis. J Dermatolog Treat. 2018;29:364-74. [ Links ] Received: October 22, 2018; Accepted: January 13, 2019 Mailing address: Valeria Aoki. E-mail: valeria.aoki@gmail.com Daniel Lorenzini. E-mail: daniellorenzini@gmail.com Conflict of interest: None. AUTHORS’ CONTRIBUTIONS Valeria Aoki 0000-0003-4256-4413 Approval of the final version of the manuscript; Conception and planning of the study; Elaboration and writing of the manuscript; Obtaining, analyzing and interpreting the data; Critical review of the literature; Critical review of the manuscript Daniel Lorenzini 0000-0002-6850-5799 Approval of the final version of the manuscript; Elaboration and writing of the manuscript; Obtaining, analyzing and interpreting the data; Critical review of the literature; Critical review of the manuscript Raquel Leão Orfali 0000-0002-2807-1404 Approval of the final version of the manuscript; Obtaining, analyzing and interpreting the data; Critical review of the literature; Critical review of the manuscript Mariana Colombini Zaniboni 0000-0002-7830-8668 Approval of the final version of the manuscript; Obtaining, analyzing and interpreting the data; Critical review of the literature; Critical review of the manuscript Zilda Najjar Prado de Oliveira 0000-0002-8596-1999 Approval of the final version of the manuscript; Critical review of the manuscript Maria Cecília Rivitti-Machado 0000-0003-2910-7330 Approval of the final version of the manuscript; Critical review of the manuscript Roberto Takaoka 0000-0003-0952-2641 Approval of the final version of the manuscript; Critical review of the manuscript Magda Blessmann Weber 0000-0001-5885-5851 Approval of the final version of the manuscript; Critical review of the manuscript Tania Cestari 0000-0003-3001-0202 Approval of the final version of the manuscript; Critical review of the manuscript Bernardo Gontijo 0000-0003-1938-5986 Approval of the final version of the manuscript; Critical review of the manuscript Andrea Machado Coelho Ramos 0000-0001-7414-3395 Approval of the final version of the manuscript; Critical review of the manuscript Claudia Marcia de Resende Silva 0000-0002-3250-1227 Approval of the final version of the manuscript; Critical review of the manuscript Silmara da Costa Pereira Cestari 0000-0001-9824-7906 Approval of the final version of the manuscript; Critical review of the manuscript Silvia Souto-Mayor 0000-0001-9335-2758 Approval of the final version of the manuscript; Critical review of the manuscript Francisca Regina Carneiro 0000-0001-6735-4004 Approval of the final version of the manuscript; Critical review of the manuscript Ana Maria Mosca de Cerqueira 0000-0001-8779-834X Approval of the final version of the manuscript; Critical review of the manuscript Cristina Laczynski 0000-0001-7483-5826 Approval of the final version of the manuscript; Critical review of the manuscript Mario Cezar Pires 0000-0001-7587-8932 Approval of the final version of the manuscript; Elaboration and writing of the manuscript; Critical review of the manuscript ©2019 by Anais Brasileiros de Dermatologia This is an Open Access article distributed under the terms of the Creative Commons Attribution NonCommercial License which permits unrestricted noncommercial use, distribution, and reproduction in any medium provided the original work is properly cited.

|
Scooped by
Gilbert C FAURE
November 12, 2018 2:35 PM
|
Abstract Background Patients with mastocytosis are at increased risk of anaphylaxis. The use of nonsteroidal anti‐inflammatory drugs (NSAIDs) is often discouraged because of this reason. However, the actual prevalence and severity of NSAID‐related hypersensitivity among patients with mastocytosis is unknown. Methods A double‐blind, placebo‐controlled acetylsalicylic acid (ASA) challenge up to a cumulative dose of 520 mg was performed among adult patients with mastocytosis. In addition, a retrospective search of the entire outpatient cohort was performed to obtain “real‐life” data on NSAID hypersensitivity. Results Fifty patients underwent an ASA challenge. Seventy percent had indolent systemic mastocytosis, 18% had mastocytosis in the skin, and 12% had advanced mastocytosis. The ASA challenge was positive in 1 patient who developed urticaria. The additional retrospective chart review revealed that 8 of 191 patients had a history of NSAID‐related hypersensitivity reaction(s), of whom 3 reported severe systemic reactions. All 8 patients had already experienced NSAID‐related hypersensitivity reactions before mastocytosis was diagnosed. Conclusions The frequency of ASA hypersensitivity was 2% in a prospective challenge study and 4.1% in a retrospective chart review of 191 patients with mastocytosis. NSAIDs can be administered safely to most patients with mastocytosis. Extra caution should be taken in patients with a history of hypersensitivity reactions to other drugs, or traditional risk factors for NSAID hypersensitivity. 1 INTRODUCTION Mastocytosis is a disease in which aberrant mast cells accumulate. The WHO recognizes different subtypes of SM.1, 2 The prevalence of anaphylaxis is higher in patients with mastocytosis compared to healthy persons.3, 4 A wide variety of stimuli can trigger mast cell degranulation and thereby lead to anaphylaxis.5 Historically, the use of certain medications that could theoretically trigger mast cell degranulation is discouraged in patients with mastocytosis. Among these are radiocontrast media, general anesthetics, opioid analgesics, and nonsteroidal anti‐inflammatory drugs (NSAIDs).3 For general anesthetics and radiocontrast media, case reports on severe (and sometimes lethal) anaphylaxis in patients with mastocytosis are available, although the absolute risk still appears low.6-8 The prevalence and severity of anaphylaxis due to NSAIDs in patients with mastocytosis is actually not known. Anxiety for NSAID‐related hypersensitivity reactions leads to confusion among both physicians and patients, and different advices between practices. The use of NSAIDs is therefore often avoided, resulting in the risk of mistreatment of patients with mastocytosis for several reasons. Firstly, these patients are at increased risk of cardiovascular morbidity, which often necessitates acetylsalicylic acid (ASA) for secondary prevention.4, 9 Secondly, ASA is a well‐known treatment for flushing in mastocytosis.10, 11 Thirdly, patients with mastocytosis relatively often suffer from various types of pain for which analgesics might be necessary.12, 13 Currently, they are often advised to only take acetaminophen which is not always sufficient. In our practice, we noted that many patients used NSAIDs uneventfully, until they received were diagnosed to have mastocytosis. Furthermore, previously performed NSAID challenges were always negative. We therefore hypothesized that the frequency and severity of NSAID‐related hypersensitivity is overestimated in patients with mastocytosis. For drug hypersensitivity in general, drug challenge tests are the gold standard diagnostic procedure.14 ASA is often used to test for general NSAID hypersensitivity because of its strong COX‐1‐inhibiting properties.15 Therefore, we designed a study using standardized drug challenge tests with ASA to investigate the exact prevalence and severity of NSAID hypersensitivity reactions in patients with mastocytosis. 2 METHODS 2.1 Patient eligibility Patients were recruited from the Mastocytosis Outpatient Clinic of the Erasmus University Medical center. All adult patients with biopsy‐proven cutaneous or systemic mastocytosis were eligible. Patients were excluded if they had a history of a prior NSAID‐related hypersensitivity reaction(s), uncontrolled asthma, rhinosinusitis, nasal polyps, active pregnancy, and high dosage of beta‐blocking drugs (equivalent to ≥100 mg of metoprolol), when they were not able to stop antihistamines or prednisolone, or were not deemed capable of handling possible delayed anaphylactic reactions at home. Preexistent mast cell mediator‐related symptoms such as pruritus or flushing were not considered to be exclusion criteria in order to represent the real‐life situation at an outpatient clinic. Moreover, flushing can be an indication for ASA. The frequency of ASA hypersensitivity among patients with mastocytosis was 2% in a prospective study and 4.1% in a retrospective cohort. NSAIDs can probably be safely administered to most patients with mastocytosis. A history of hypersensitivity reactions to other drugs might increase the risk of NSAID hypersensitivity among patients with mastocytosis. 2.2 Study protocol All patients underwent a double‐blind, placebo‐controlled ASA challenge in a randomized order. The minimum interval between the two test days was 14 days. The study medication was provided in a blinded fashion by the pharmacy of the Erasmus MC. Patients had to stop H1‐antagonists and leukotriene antagonists for 3 days prior to the drug challenge. The challenge took place at the Allergy Outpatient Clinic. Patients received three incremental doses of ASA of 40, 80, and 400 mg (or matched placebo tablets), leading to a cumulative dose of 520 mg. The interval between each dose was 1 hour, and patients were observed for an additional 2 hours after the administration of the third dose. Mast cell mediator‐related symptoms were systematically scored before the start of each drug challenge and after 1, 2, and 4 hours. We used an adapted form of the scoring system for food challenges as proposed by Grabenhenrich et al.16 This form scores symptoms according to organ system and severity and is in our practice routinely used for both food and drug challenges. In addition, numeric rating scale (NRS) score was obtained for mast cell mediator‐related symptoms such as pruritus and headache. An increase of 3 points in this scale during the challenge was considered significant. All challenges were conducted and assessed by MH and/or SdV. In cases of doubt, a second investigator (RGvW or PvD) was consulted to assess the symptoms. Deblinding of the investigators and patients took place 24 hours after all 50 patients completed both challenge days. 2.3 Outcomes and definitions The ASA challenge was considered positive when a patient developed objective mast cell mediator‐related symptoms within 12 hours after the administration of the third dose on the day they received the verum, and had no symptoms on the placebo day. The challenge was considered negative when a patient developed no symptoms on the verum day, regardless of any symptoms on the placebo day. The WHO criteria were used to define the subtype of mastocytosis.1 Patients with maculopapular cutaneous mastocytosis (MPCM) who never underwent bone marrow biopsy, or had negative bone marrow investigation, were categorized as mastocytosis in the skin (MIS). An atopic background was defined as a history of atopic dermatitis, asthma, rhinoconjunctivitis, and/or positive specific IgE for inhalation or food allergens. Next to atopy, other traditional risk factors for NSAID hypersensitivy were defined as the presence of asthma, nasal polyps, and chronic rhinosinusitis. Eosinophilia was defined as an absolute eosinophil count of >500 × 106 in peripheral blood. 2.4 Additional retrospective cohort study Next to the prospective challenge study, we retrospectively searched the electronic patient records of all adult patients who visited the mastocytosis center from January 2009 until January 2017 and who fulfilled the criteria for mastocytosis in the skin (MIS), cutaneous or systemic mastocytosis (SM). Patients who already participated in the challenge study were excluded from the retrospective cohort. Patients with a history of NSAID‐related hypersensitivity reactions, or patients who had proven NSAID tolerance prior to the start of this study, were subsequently contacted to obtain further clinical details. NSAID tolerance was considered as proven when a drug challenge was negative or when (accidental) NSAID ingestion was uneventful after the diagnosis of mastocytosis was made. The characteristics of the patients with and without NSAID tolerance from this retrospective cohort were compared to identify possible differences. 2.5 Ethical considerations This trial was performed according to the latest Helsinki guidelines. The study was approved by the local medical ethics committee. All participants provided written informed consent. The trial was registered in the EudraCT database, Number 2015‐004604‐37. 2.6 Statistical analysis We used IBM SPSS 21 for all analyses. Patient characteristics were noted as median with interquartile range (IQR) for continuous variables and as the number with percentage for dichotomous variables. To calculate potential differences between the groups with and without NSAID hypersensitivity, the Mann‐Whitney U test was used for continuous variables and the chi‐square test for dichotomous variables. 2.7 Power calculation The prevalence of NSAID‐related hypersensitivity reactions in the general population is <1%. We hypothesized that the risk in patients with systemic mastocytosis is only marginally higher than in the general population. With an estimated frequency of allergic reactions of 4% in our study population, inclusion of 50 subjects in total will lead to a 95% confidence interval of 1%‐13%. The estimated frequency was based on self‐reported reactions by patients in the Erasmus MC cohort combined with circumstantial data of other cohort studies on mastocytosis (see Discussion section for references). 3 RESULTS 3.1 Study population At the moment of inclusion in April 2017, 173 patients were considered eligible to participate in the trial (Figure 1), and 58 patients signed the informed consent. After inclusion, 8 patients dropped out before the ASA challenge was performed completely. One of these patients was excluded after the first challenge day because she started to use prednisolone for arthritis which was not related to the trial. Two patients experienced anaphylactoid reactions related to their mastocytosis within days after cessation of the antihistamines. The other patients withdrew consent. The inclusion process was stopped after 50 subjects completed both days of the challenge. The final study population thus consisted of 50 patients. The median age was 55 years, and most participants had indolent SM (Table 1). Age in years, median (IQR) 55 (16) Male, n (%) 16 (32) Subtype according to WHO criteria 1, 2, n (%) MIS 9 (18)a ISM With skin lesions 26 (52) Without skin lesions 9 (18) SSM 3 (6) SM‐AHN 2 (4) ASM 1 (2) Serum tryptase level at diagnosis in μg/L, median (IQR) 25.0 (17.8) Atopic background, n (%) 13 (26) Eosinophilia, n (%) 2 (4) Previous hypersensitivity reaction to any drug, n (%) 5 (10) Previous anaphylaxis due to any trigger, n (%) 23 (46) Wasp 7 (30) Unknown 7 (30) Physical stimuli 4 (17) Other drugsb 5 (22) Miscellaneousc 6 (26) ASM, aggressive systemic mastocytosis; IQR, interquartile range; ISM, indolent systemic mastocytosis; MIS, mastocytosis in the skin; SSM, smoldering systemic mastocytosis; SM‐AHN, systemic mastocytosis with associated hematological neoplasm. a One patient underwent incomplete bone marrow investigation, and 8 patients declined bone marrow punction. b Other drugs: proton pump inhibitor (1×), morphine (2×), penicillin (1×), and codeine (1×). c Miscellaneous triggers: horsefly (n = 2), jellyfish sting (n = 1), fire ant (n = 1), anesthesia (n = 1), codeine (n = 1). 3.2 Results of ASA challenge The challenge was positive in one patient (2%), who developed an urticarial rash 4 hours after ingestion of the third dose of ASA, which correspond s with a cumulative dose of 520 mg. The rash subsided after she took 10 mg of hydroxyzine. This patient had smoldering SM based on a serum tryptase level of ≥200 μg/L and hepatosplenomegaly. She had never used NSAIDs before. She had previously developed a rash after the administration of radiocontrast media and reported an increase in mast cell mediator‐related symptoms after the consumption of alcoholic beverages and histamine‐rich food. Three other patients reported subjective symptoms on the day of the verum but not on the day they received placebo. These symptoms consisted of mild flushing in 1 patient, generalized pruritus in 1 patient, and lightheadedness in 1 patient. The flushing was not considered as a positive challenge because it occurred after the second dose of 80 mg and subsided spontaneously despite the fact that the next increasing dose of ASA was administered according to protocol. Moreover, this patient has spontaneous flushes multiple times a week. Similarly, the patient with pruritus already had pruritus before the start of the challenge and the NRS score increased by 2 points throughout the day of the challenge which was below the prespecified threshold of 3 points (see Methods section). The last patient experienced lightheadedness 15 minutes after the ingestion of the third dose of ASA, but had no other mast cell mediator‐related symptoms and stable vital parameters. The serum tryptase level did not increase as compared to a baseline measurement. Seven patients had a reaction on the placebo day, of whom 1 had objective macular erythema on the arms and trunk and the other 6 patients had subjective symptoms. Four patients who had a reaction to placebo already had mast cell mediator‐related symptoms at the start of the challenge, consisting of pruritus and/or flushing. 3.3 Patients with a history of NSAID‐related reactions In addition to the challenge study, we retrospectively searched the electronic records of all adult patients with mastocytosis that visited the Erasmus MC from 2009 until 2017. Of a total of 191 patients, 8 patients had an annotation of “NSAID allergy” in their medical record. This results in a prevalence of self‐reported NSAID‐related hypersensitivity of 4.1% in our entire cohort. Fifteen patients had proven NSAID tolerance prior to this study. Table 2 summarizes the clinical characteristics of these patients. Age at diagnosis (years) Sex Subtype Skin involvement Serum tryptase at diagnosis (μg/L) Atopy Type of NSAID Timing of reaction Symptoms of reaction MC mediator‐related reaction to other stimuli 1 41 F CMa MPCM 8.6 Yes ASAb 80 mg 2 h Angioedema Penicillin, lidocaine 2 72 M ISM No 20.0 No Ibuprofen Diclofenacc Unknown Generalized pruritus, blurry vision – 3 51 F ISM MPCM 125.0 Yes Ibuprofen Diclofenacc 5 min Angioedema, palpitations, collapsed Heat 4 62 F ISM MPCM 118.0 No Diclofenac Unknown Angioedema Alcohol consumption 5 42 M ISM MPCM 31.4 No Naproxen 10 min Erythema, stridor, hypotensiond Alcohol consumption, wasp sting, temperature changes (cold) 6 40 M ISM No 24.7 Yes Acetaminophen 1000 mg 5 min Diffuse erythema Morphine, strong odors 7 48 F ISM MPCM 43.5 Naproxen 5 min Diffuse erythema – 8 68 F ISM MPCM 17.8 No Diclofenac 20 min Hypotension, collapsed Iodated contrast media ASA, acetylsalicylic acid; CM, cutaneous mastocytosis; MPCM, maculopapular cutaneous mastocytosis; NSAID, nonsteroidal anti‐inflammatory drug. a Complete workup with bone marrow investigation was negative for mastocytosis. b Hymenoptera sensitization could not be confirmed by specific IgE nor intradermal tests. c Two separate reactions at different occurrences. d Treatment at emergency department required. All patients had experienced NSAID‐related reactions before they received the diagnosis of mastocytosis. The most frequent symptoms were angioedema, erythema, and hypotension. Three patients required treatment in an emergency department. Of the patients who could reliably recall their reaction at the time of questioning, everyone experienced a reaction within 2 hours after the ingestion of the NSAID. Patient number 5 developed a reaction after the combination of naproxen and a wasp sting. Hymenoptera sensitization could not be confirmed. Although it is likely that Hymenoptera was the main culprit and naproxen acted as a cofactor, the patient was labeled as “NSAID intolerant” because of the severity of the reaction and the risk of aggravation of future reactions with the use of NSAIDs. Patient number 3 later had a drug challenge with celecoxib which was negative. Patient numbers 6 and 8 both later had a negative drug challenge with naproxen, which excludes a general nonspecific NSAID hypersensitivity. Notably, seven patients (87.5%) reported mast cell mediator‐related reactions to physical stimuli. These reactions ranged from flushing or gastrointestinal symptoms to anaphylaxis. 3.4 Characteristics associated with NSAID hypersensitivity As only one patient had a positive ASA challenge, the data from the prospective study could not be used to reliably identify any potential clinical characteristics that are associated with NSAID hypersensitivity. Therefore, the data from the retrospective cohort were analyzed for this purpose (Table 3). No NSAID hypersensitivity (n = 15) NSAID hypersensitivity (n = 8) P‐value Age, median (IQR) 51 (20) 49 (25) >.10 Male sex, n (%) 6 (40) 3 (33.3) >.10 Subtype, n (%) MIS 5a (33.3) 1b (12.5) >.10 ISM 10 (66.7) 7 (87.5) SSM 0 0 SM‐AHN 0 0 ASM 0 0 Presence of skin mastocytosis, n (%) 11 (73.3) 5 (62.5) >.10 Serum tryptase at diagnosis, median (IQR) 28.2 (10.9)c Range: 2.2‐72.0 28 (53)c Range: 8.6‐125.0 >.10 History of anaphylaxis, n (%) 5 (33.3) 4 (50) >.10 Pruritus, n (%)d 7 (46.7) 0 .021 Flushing, n (%)d 5 (33.3) 1 (12.5) >.10 Dyspepsia, n (%)d 3 (10.3) 1 (12.5) >.10 Diarrhea, n (%)d 3 (20) 0 >.10 Fatigue, n (%)d 6 (42.9) 1 (12.5) >.10 Subjective cognitive problems, n (%) 4 (40) 0 .074 Osteoporosis, n (%) 1 (7.1) 2 (28.6) >.10 Eosinophilia, n (%) 2 (13.3) 3 (37.5) >.10 Atopy, n (%) 4 (26.7) 3 (37.5) >.10 History of hypersensitivity reaction to other drugs, n (%)e 1 (6.7) 3 (37.5) .063 Alcohol intolerance, n (%)f 4/5 (44.4) 4/5 (80) >.10 MC mediator‐related reaction to physical triggers, n (%)c 5/8 (62.5) 4/5 (80) >.10 a Bone marrow investigation was incomplete in 1 patient and not performed in the other patients. b Bone marrow investigation negative, classifying this patient as cutaneous mastocytosis. c Physical triggers: heat, cold, stress, exercise. d Symptom present ≥3 d per week. e The culprit drug was amoxicillin in the NSAID‐tolerant patient. See Table 2 for the culprit drugs in the NSAID hypersensitivity group. f Not known for all patients because some patients never consume alcohol. Overall, patients with NSAID tolerance appear to have more daily mast cell mediator‐related symptoms such as flushing, cognitive problems, fatigue, and pruritus. Only the latter was statistically significant (P = .021, chi‐square test), probably due to the small numbers of patients. Patients with NSAID hypersensitivity reported more reactions to other drugs, although this difference did not reach statistical significance (P = .063). The same accounted for peripheral blood eosinophilia (P = .181), osteoporosis (P = .186), and alcohol intolerance (P = .308). Strikingly, traditional risk factors for NSAID hypersensitivity in the general population such as atopy, asthma, or rhinitis were not more frequent in the NSAID hypersensitivity group of our cohort. Neither were there any relevant differences in age, sex, serum tryptase levels, or skin involvement of mastocytosis. 4 DISCUSSION This is the first double‐blind, placebo‐controlled challenge study to investigate the prevalence and severity of ASA hypersensitivity among patients with mastocytosis. Only 1 of 50 participants (2%) had a positive ASA challenge, consisting of an urticarial rash. Three other patients had subjective symptoms to ASA. The characteristics of the study population were overall representative of a patient cohort in a tertiary center for mastocytosis, except for a relatively low number of male participants.17, 18, 13 However, the exclusion of patients with known risk factors for NSAID hypersensitivity might have led to a selection bias. Therefore, we performed an additional retrospective analysis of our entire cohort of 191 patients. This resulted in a prevalence of self‐reported NSAID‐related hypersensitivity of 4.1%. Importantly, all patients with NSAID hypersensitivity experienced one or more reactions before the diagnosis of mastocytosis was established. Although interpretation of any differences between the patients with and without NSAID hypersensitivity is difficult due to the small patient numbers, it appears from the retrospective cohort that patients with NSAID hypersensitivity more often experienced hypersensitivity reactions to other drugs and/or alcohol. It must be noted that three patients reported a hypersensitivity reaction to amoxicillin, which is the third most reported culprit for drug‐related reactions in the Netherlands. We cannot exclude that the relationship between amoxicillin and the reported reactions was based on coincidence. Another notable difference is the higher prevalence of mast cell mediator‐related symptoms among NSAID‐tolerant patients. Possibly, this difference represents the clinical practice, because patients with symptoms such as flushing are more often in need for ASA and therefore were more likely to undergo an NSAID challenge out of medical necessity. A causal explanation seems unlikely. Interestingly, 2 of 8 patients with a history of NSAID‐related hypersensitivity reactions later had negative unblinded challenges with another NSAID. This can be explained in multiple ways: They might have a specific, IgE‐mediated allergy to the culprit NSAID. Another, more likely, explanation is the fact that NSAIDs can be a cofactor to augment anaphylaxis.19, 20 The current trial does not provide prospective data on the role of NSAIDs as a cofactor in patients with mastocytosis. Also, although the use of ASA as a model for general NSAID hypersensitivity is widely accepted, a specific allergy for one type of NSAID is potentially missed with this approach. Moreover, the currently presented data cannot be extrapolated to patients with traditional risk factors for NSAID hypersensitivity, such as asthma, nasal polyposis, or atopic constitution. However, most patients with mastocytosis do not have such risk factors21; thus, most patients would fulfill the currently used inclusion criteria. Lastly, a possible caveat of drug challenges in patients with mastocytosis is the fact that many of them already have mast cell mediator‐related symptoms on a daily basis, especially as anti‐mediator medications need to be interrupted prior to a challenge. Using NRS scores, we tried to score these symptoms as objectively as possible; however, some degree of bias in the assessment of challenge studies cannot be excluded in this patient category. The prevalence of NSAID hypersensitivity in our cohort of patients with mastocytosis is only slightly higher compared to the prevalence of 1%‐2% of self‐reported NSAID‐related hypersensitivity in a general population.22 There are few comprehensive data on NSAID hypersensitivity among patients with mastocytosis published to date. One retrospective study described 20 patients who received ASA in varying dosages and schedules. Two patients (10%) reported a mild reaction: either delayed urticaria or immediate flushing.10 Other descriptive population studies reported a prevalence ranging between 2.3% and 6%, of mostly mild immediate‐type reactions.20, 23, 24 We could not find published proof of fatal anaphylaxis due to NSAIDs in patients with mastocytosis. Conversely, in a population of 137 persons with drug‐ or food‐related anaphylaxis, mastocytosis was found in only 2 patients.25 Moreover, there was no association between NSAID hypersensitivity and serum tryptase levels in a general cohort.26 An EAACI position paper advises that patients with mastocytosis and known NSAID tolerance can safely keep taking NSAIDs, but all others should undergo workup.27 However, that workup is not further specified in this study. Based on our current results, we suggest that everyone with mastocytosis who has never experienced a hypersensitivity reaction to NSAIDs, or other drugs, can safely start taking NSAIDs at home. Given the fact that patients who have experienced a hypersensitivity reaction to another drug appear to be at a higher risk of NSAID hypersensitivity reactions, it seems appropriate to administer the first dose in a clinical setting, preferably with an incremental challenge protocol. As mentioned before, the interpretation of such challenges is very delicate and requires experience in this area. Despite the placebo‐controlled approach, some patients will have only subjective symptoms to an NSAID, and although this is likely to be a “placebo reaction,” it cannot be excluded that these minor symptoms reflect some reaction to the NSAID. The risk of developing more serious reactions in the future is unclear for these patients, and careful counseling is of paramount importance. Possibly, patients with a history of NSAID‐related hypersensitivity reactions can also be challenged with another NSAID. Depending on the type and severity of the previous reaction(s), it can be safer to challenge with a selective COX‐2 inhibitor in these cases. Unfortunately, our study cannot corroborate this suggestion. Prospective placebo‐controlled studies on these topics would be highly interesting, although are hindered by potential safety issues. On a final note, although the possible benefits of ASA and NSAIDs in general for patients with mastocytosis are clear, the indication must be weighed against possible adverse effects. For instance, patients with mastocytosis already are at risk of peptic ulcer disease,28 which might be increased by the use of NSAIDs. Moreover, it is well‐known that NSAIDs can act as a cofactor in anaphylaxis. Ultimately, a careful consideration of risks and benefits needs to be made for each individual, and patients should be consulted on the possible risks. 5 CONCLUSIONS In summary, the frequency of NSAID hypersensitivity among patients with mastocytosis was 2%, as determined by a prospective double‐blind ASA challenge. The frequency of self‐reported NSAID hypersensitivity in a retrospective cohort was 4.1%. Based on the mild reactions we saw in our study, combined with the real‐life experience that all patients with severe NSAID hypersensitivity experienced these reactions prior to their diagnosis of mastocytosis, we conclude that it is safe to administer NSAIDs to most patients with mastocytosis if they do not have a history of prior NSAID hypersensitivity reactions. Extra caution might be taken in patients with previous hypersensitivity reactions to other drugs, or with traditional risk factors for NSAID hypersensitivity. ACKNOWLEDGMENTS We would like to thank N.W. de Jong, PhD (Dept. of Allergy, Erasmus MC), for her assistance with the practical organization of the challenge study, and M.S. van Maaren, MD (Dept. of Allergy, Erasmus MC), for providing and advising on the scoring system for the ASA challenges. CONFLICTS OF INTEREST The authors declare that they have no conflicts of interest. AUTHORS’ CONTRIBUTIONS MH and PvD are responsible for the concept and design of the study. MH and SdV performed the inclusion and drug challenges and collected and analyzed the data. PvH and RGvW gave advice about the design of the trial and interpretation of the data. All authors revised the manuscript on multiple occasions. REFERENCES Notes : Trial registration: This trial was registered in the EudraCT database, Number 2015‐004604‐37. ASM, aggressive systemic mastocytosis; IQR, interquartile range; ISM, indolent systemic mastocytosis; MIS, mastocytosis in the skin; SSM, smoldering systemic mastocytosis; SM‐AHN, systemic mastocytosis with associated hematological neoplasm. 3 One patient underwent incomplete bone marrow investigation, and 8 patients declined bone marrow punction. 4 Other drugs: proton pump inhibitor (1×), morphine (2×), penicillin (1×), and codeine (1×). 5 Miscellaneous triggers: horsefly (n = 2), jellyfish sting (n = 1), fire ant (n = 1), anesthesia (n = 1), codeine (n = 1). ASA, acetylsalicylic acid; CM, cutaneous mastocytosis; MPCM, maculopapular cutaneous mastocytosis; NSAID, nonsteroidal anti‐inflammatory drug. 7 Complete workup with bone marrow investigation was negative for mastocytosis. 8 Hymenoptera sensitization could not be confirmed by specific IgE nor intradermal tests. 9 Two separate reactions at different occurrences. 10 Treatment at emergency department required. 11 Bone marrow investigation was incomplete in 1 patient and not performed in the other patients. 12 Bone marrow investigation negative, classifying this patient as cutaneous mastocytosis. 13 Physical triggers: heat, cold, stress, exercise. 14 Symptom present ≥3 d per week. 15 The culprit drug was amoxicillin in the NSAID‐tolerant patient. See Table 2 for the culprit drugs in the NSAID hypersensitivity group. 16 Not known for all patients because some patients never consume alcohol.

|
Scooped by
Gilbert C FAURE
October 28, 2018 5:52 AM
|
The updosing of second-generation antihistamines for chronic urticaria is based on inconsistent findings.Herein, we report data on the treatment of children with chronic spontaneous urticaria (CSU) u...

|
Scooped by
Gilbert C FAURE
September 18, 2018 12:05 PM
|
Download PDF William D. Chey, MD, FACG1, Grigorios I. Leontiadis, MD, PhD2, Colin W. Howden, MD, FACG3 and Steven F. Moss, MD, FACG4 1Division of Gastroenterology, University of Michigan Health System, Ann Arbor, Michigan, USA; 2Division of Gastroenterology, McMaster University, Hamilton, Ontario, Canada; 3Division of Gastroenterology, University of Tennessee Health Science Center, Memphis, Tennessee, USA; and 4Division of Gastroenterology, Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA Am J Gastroenterol 2017; 112: 212–238; doi:10.1038/ajg.2016.563; published online 10 January 2017 Received 28 June 2016; accepted 7 October 2016 Correspondence: William D. Chey, MD, FACG, Timothy T. Nostrant Professor of Gastroenterology and Nutrition Sciences, Division of Gastroenterology, University of Michigan Health System, 3912 Taubman Center, SPC 5362, Ann Arbor, Michigan 49109-5362, USA. E-mail: wchey@umich.edu Abstract Helicobacter pylori (H. pylori) infection is a common worldwide infection that is an important cause of peptic ulcer disease and gastric cancer. H. pylori may also have a role in uninvestigated and functional dyspepsia, ulcer risk in patients taking low-dose aspirin or starting therapy with a non-steroidal anti-inflammatory medication, unexplained iron deficiency anemia, and idiopathic thrombocytopenic purpura. While choosing a treatment regimen for H. pylori, patients should be asked about previous antibiotic exposure and this information should be incorporated into the decision-making process. For first-line treatment, clarithromycin triple therapy should be confined to patients with no previous history of macrolide exposure who reside in areas where clarithromycin resistance amongst H. pylori isolates is known to be low. Most patients will be better served by first-line treatment with bismuth quadruple therapy or concomitant therapy consisting of a PPI, clarithromycin, amoxicillin, and metronidazole. When first-line therapy fails, a salvage regimen should avoid antibiotics that were previously used. If a patient received a first-line treatment containing clarithromycin, bismuth quadruple therapy or levofloxacin salvage regimens are the preferred treatment options. If a patient received first-line bismuth quadruple therapy, clarithromycin or levofloxacin-containing salvage regimens are the preferred treatment options. Details regarding the drugs, doses and durations of the recommended and suggested first-line and salvage regimens can be found in the guideline. Introduction Helicobacter pylori infection remains one of the most common chronic bacterial infections affecting humans. Since publication of the last American College of Gastroenterology (ACG) Clinical Guideline in 2007, significant scientific advances have been made regarding the management of H. pylori infection. The most significant advances have been made in the arena of medical treatment. Thus, this guideline is intended to provide clinicians working in North America with updated recommendations on the treatment of H. pylori infection. For the purposes of this document, we have defined North America as the United States and Canada. Whenever possible, recommendations are based upon the best available evidence from the world’s literature with special attention paid to literature from North America. When evidence from North America was not available, recommendations were based upon data from international studies and expert consensus. This guidance document was developed using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) system (1), which provides a level of evidence and strength of recommendation for statements developed using the PICO (patient population, intervention or indicator assessed, comparison group, outcome achieved) format. At the start of the guideline development process, the authors developed PICO questions relevant to Helicobacter pylori infection. The authors worked with research methodologists from McMaster University to conduct focused literature searches to provide the best available evidence to address the PICO questions. Databases searched included MEDLINE, EMBASE and Cochrane CENTRAL from 2000 to 11 September 2014. Search terms included “pylori, treat*, therap*, manag*, eradicat*”. The full literature search strategy is provided as Supplementary Appendix 1 online. After assessing the risk of bias, indirectness, inconsistency, and imprecision, the level of evidence for each recommendation was reported as “high” (further research is unlikely to change the confidence in the estimate of effect), “moderate” (further research would be likely to have an impact on the confidence in the estimate of effect), “low” (further research would be expected to have an impact on the confidence in the estimate of effect), or “very low” (any estimate of effect is very uncertain). The strength of recommendations was determined to be “strong” or “conditional” based on the quality of evidence, the certainty about the balance between desirable and undesirable effects of the intervention, the certainty about patients’ values and preferences, and the certainty about whether the recommendation represents a wise use of resources. A summary of the recommendation statements for this management guideline is provided in Table 1. The justification for the assessments of the quality of evidence for each statement can be found in Supplementary Appendix 2 online. Table 1. Recommendation statements What is known about the epidemiology of H. pylori infection in North America? Which are the high risk groups? H. pylori infection is chronic and is usually acquired in childhood. The exact means of acquisition is not always clear. The incidence and prevalence of H. pylori infection are generally higher among people born outside North America than among people born here. Within North America, the prevalence of the infection is higher in certain racial and ethnic groups, the socially disadvantaged, and people who have immigrated to North America (Factual statement, low quality of evidence). What are the indications to test for, and to treat, H. pylori infection? Since all patients with a positive test of active infection with H. pylori should be offered treatment, the critical issue is which patients should be tested for the infection (strong recommendation; quality of evidence not applicable). All patients with active peptic ulcer disease (PUD), a past history of PUD (unless previous cure of H. pylori infection has been documented), low-grade gastric mucosa-associated lymphoid tissue (MALT) lymphoma, or a history of endoscopic resection of early gastric cancer (EGC) should be tested for H. pylori infection. Those who test positive should be offered treatment for the infection (Strong recommendation; quality of evidence: high for active or history of PUD, low for MALT lymphoma, low for history of endoscopic resection of EGC). In patients with uninvestigated dyspepsia who are under the age of 60 years and without alarm features, non-endoscopic testing for H. pylori infection is a consideration. Those who test positive should be offered eradication therapy (conditional recommendation; quality of evidence: high for efficacy, low for the age threshold). When upper endoscopy is undertaken in patients with dyspepsia, gastric biopsies should be taken to evaluate for H. pylori infection. Infected patients should be offered eradication therapy (strong recommendation; high quality of evidence). Patients with typical symptoms of gastroesophageal reflux disease (GERD) who do not have a history of PUD need not be tested for H. pylori infection. However, for those who are tested and found to be infected, treatment should be offered, acknowledging that effects on GERD symptoms are unpredictable (strong recommendation; high quality of evidence). In patients taking long-term, low-dose aspirin, testing for H. pylori infection could be considered to reduce the risk of ulcer bleeding. Those who test positive should be offered eradication therapy to reduce the risk of ulcer bleeding (conditional recommendation; moderate quality of evidence). Patients initiating chronic treatment with a non-steroidal anti-inflammatory drug (NSAID) should be tested for H. pylori infection. Those who test positive should be offered eradication therapy (Strong recommendation; Moderate quality of evidence). The benefit of testing and treating H. pylori in a patient already taking an NSAID remains unclear (conditional recommendation; low quality of evidence). Patients with unexplained iron deficiency anemia despite an appropriate evaluation should be tested for H. pylori infection. Those who test positive should be offered eradication therapy (conditional recommendation; low quality of evidence). Adults with idiopathic thrombocytopenic purpura (ITP) should be tested for H. pylori infection. Those who test positive should be offered eradication therapy (conditional recommendation; very low quality of evidence). There is insufficient evidence to support routine testing for and treatment of H. pylori in asymptomatic individuals with a family history of gastric cancer or patients with lymphocytic gastritis, hyperplastic gastric polyps, and hyperemesis gravidarum (no recommendation; very low quality of evidence). What are evidence-based first-line treatment strategies for providers in North America? Patients should be asked about any previous antibiotic exposure(s) and this information should be taken into consideration when choosing an H. pylori treatment regimen (conditional recommendation; moderate quality of evidence). Clarithromycin triple therapy consisting of a PPI, clarithromycin, and amoxicillin or metronidazole for 14 days remains a recommended treatment in regions where H. pylori clarithromycin resistance is known to be <15% and in patients with no previous history of macrolide exposure for any reason (Conditional recommendation; low quality of evidence (for duration: moderate quality of evidence)). Bismuth quadruple therapy consisting of a PPI, bismuth, tetracycline, and a nitroimidazole for 10–14 days is a recommended first-line treatment option. Bismuth quadruple therapy is particularly attractive in patients with any previous macrolide exposure or who are allergic to penicillin (strong recommendation; low quality of evidence). Concomitant therapy consisting of a PPI, clarithromycin, amoxicillin and a nitroimidazole for 10–14 days is a recommended first-line treatment option (strong recommendation; low quality of evidence (for duration: very low quality of evidence)). Sequential therapy consisting of a PPI and amoxicillin for 5–7 days followed by a PPI, clarithromycin, and a nitroimidazole for 5–7 days is a suggested first-line treatment option (conditional recommendation; low quality of evidence (for duration: very low quality of evidence)). Hybrid therapy consisting of a PPI and amoxicillin for 7 days followed by a PPI, amoxicillin, clarithromycin and a nitroimidazole for 7 days is a suggested first-line treatment option (conditional recommendation; low quality of evidence (For duration: very low quality of evidence)). Levofloxacin triple therapy consisting of a PPI, levofloxacin, and amoxicillin for 10–14 days is a suggested first-line treatment option (conditional recommendation; low quality of evidence (For duration: very low quality of evidence)). Fluoroquinolone sequential therapy consisting of a PPI and amoxicillin for 5–7 days followed by a PPI, fluoroquinolone, and nitroimidazole for 5–7 days is a suggested first-line treatment option (conditional recommendation; low quality of evidence (for duration: very low quality of evidence)). What factors predict successful eradication when treating H. pylori infection? The main determinants of successful H. pylori eradication are the choice of regimen, the patient’s adherence to a multi-drug regimen with frequent side-effects, and the sensitivity of the H. pylori strain to the combination of antibiotics administered (Factual statement; moderate quality of evidence). What do we know about H. pylori antimicrobial resistance in the North America? Data regarding antibiotic resistance among H. pylori strains from North America remains scarce. Organized efforts are needed to document local, regional, and national patterns of resistance in order to guide the appropriate selection of H. pylori therapy (strong recommendation; low quality of evidence). What methods can be used to evaluate for H. pylori antibiotic resistance and when should testing be performed? Although H. pylori antimicrobial resistance can be determined by culture and/or molecular testing, (strong recommendation; moderate quality of evidence), these tests are currently not widely available in the United States. Should we test for teatment success after H. pylori eradication therapy? Whenever H. pylori infection is identified and treated, testing to prove eradication should be performed using a urea breath test, fecal antigen test or biopsy-based testing at least 4 weeks after the completion of antibiotic therapy and after PPI therapy has been withheld for 1–2 weeks. (Strong recommendation; Low quality of evidence (for the choice of methods to test for eradication: Moderate quality of evidence)). When first-line therapy fails, what are the options for salvage therapy? In patients with persistent H. pylori infection, every effort should be made to avoid antibiotics that have been previously taken by the patient (unchanged from previous ACG guideline (1)) (Strong recommendation; moderate quality of evidence). Bismuth quadruple therapy or levofloxacin salvage regimens are the preferred treatment options if a patient received a first-line treatment containing clarithromycin. Selection of best salvage regimen should be directed by local antimicrobial resistance data and the patient’s previous exposure to antibiotics (Conditional recommendation; for quality of evidence see individual statements below). Clarithromycin or levofloxacin-containing salvage regimens are the preferred treatment options, if a patient received first-line bismuth quadruple therapy. Selection of best salvage regimen should be directed by local antimicrobial resistance data and the patient’s previous exposure to antibiotics (Conditional recommendation; for quality of evidence see individual statements below). The following regimens can be considered for use as salvage treatment: Bismuth quadruple therapy for 14 days is a recommended salvage regimen. (Strong recommendation; low quality of evidence) Levofloxacin triple regimen for 14 days is a recommended salvage regimen. (Strong recommendation; moderate quality of evidence (For duration: low quality of evidence) Concomitant therapy for 10–14 days is a suggested salvage regimen. (conditional recommendation; very low quality of evidence) Clarithromycin triple therapy should be avoided as a salvage regimen. (conditional recommendation; low quality of evidence) Rifabutin triple regimen consisting of a PPI, amoxicillin, and rifabutin for 10 days is a suggested salvage regimen (conditional recommendation; moderate quality of evidence (For duration: very low quality of evidence)). High-dose dual therapy consisting of a PPI and amoxicillin for 14 days is a suggested salvage regimen (conditional recommendation; low quality of evidence (For duration: very low quality of evidence)). When should penicillin allergy testing be considered in patients with H. pylori infection? Most patients with a history of penicillin allergy do not have true penicillin hypersensitivity. After failure of first-line therapy, such patients should be considered for referral for allergy testing since the vast majority can ultimately be safely given amoxicillin-containing salvage regimens (strong recommendation; Low quality of evidence). Question 1: What Is Known About the Epidemiology of H. pylori Infection in North America? Which Are the High-Risk Groups? Recommendation H. pylori infection is chronic and is usually acquired in childhood. The exact means of acquisition is not always clear. The incidence and prevalence of H. pylori infection are generally higher among people born outside North America than among people born here. Within North America, the prevalence of the infection is higher in certain racial and ethnic groups, the socially disadvantaged, and people who have immigrated to North America (factual statement, low quality of evidence). H. pylori infection is usually acquired during childhood (2, 3, 4, 5, 6) although the exact means of acquisition is not always clear. Risk factors for acquiring the infection include low socioeconomic status (6, 7, 8) increasing number of siblings (9) and having an infected parent—especially an infected mother (10). In the Ulm (Germany) Birth Cohort Study, the odds ratio (OR) for acquiring H. pylori infection if a child’s mother was infected was 13.0 (95% confidence interval (CI) 3.0–55.2) (10) Apart from intra-familial spread, the infection may also be transmitted through contaminated water supplies (11) particularly in developing countries. Although infection rates for male and female children are similar (3, 12) there may be a slight male preponderance of the infection in adulthood. In a meta-analysis of observational, population-based studies, men were slightly more likely to be H. pylori-positive than women; OR=1.16 (95% CI 1.11–1.22) (12) This was confirmed in a study of adults in Ontario, Canada, in which the overall seroprevalence was 23.1% but higher in men (29.4%) than women (14.9%) (13). One explanation that has been proposed for the lower seroprevalence in women is that they may be more likely to clear H. pylori infection because of higher rates of incidental antibiotic use for other indications (12). There is evidence for a birth cohort effect on H. pylori prevalence; for example, people who were born in the 1930s are more likely to have been infected during childhood than people born in the 1960s. In a study conducted among 7310 US veterans with gastrointestinal symptoms, seroprevalence was 73% among those born before 1920 and 22% in those born after 1980 (14). The overall prevalence of the infection in these US veterans fell from 70.8% in 1997 to a plateau of around 50% after 2002. Within North America, the prevalence of H. pylori infection varies with socioeconomic status and race/ethnicity (14, 15, 16, 17). In general, the prevalence is lower among non-Hispanic whites than among other racial/ethnic groups including African Americans, Hispanic Americans, Native Americans, and Alaska natives (5, 14, 15, 18). African Americans with a higher proportion of African ancestry have been reported to have higher rates of H. pylori infection than African Americans with a lower proportion of African ancestry suggesting that racial/genetic factors may have some role in predisposition to the infection unrelated to socioeconomic factors (16). Higher prevalence rates have been found among those living close to the US/Mexico border (19, 20); in one study (19), prevalence of H. pylori assessed by stool antigen testing was 38.2%. Prevalence has also been reported to be high among Alaska natives (18) and Canadian First Nations populations (21). The prevalence of H. pylori infection is generally lower in the United States than in many other parts of the world, particularly in comparison to Asia and Central and South America (8, 22). There is, however, preliminary evidence that it may be falling in some previously high prevalence areas (22). People immigrating to North America from Asia and other parts of the world have a much higher prevalence of the infection than people born in North America (23). In one study, the seroprevalence among immigrants from East Asia was 70.1% (24). Hispanic immigrants to North America have higher rates of the infection than first- or second-generation Hispanics who were born here (25). Question 2: What Are the Indications to Test For, and to Treat, H. pylori Infection? Recommendations Since all patients with a positive test of active infection with H. pylori should be offered treatment, the critical issue is which patients should be tested for the infection (strong recommendation, quality of evidence: not applicable), All patients with active peptic ulcer disease (PUD), a past history of PUD (unless previous cure of H. pylori infection has been documented), low-grade gastric mucosa-associated lymphoid tissue (MALT) lymphoma, or a history of endoscopic resection of early gastric cancer (EGC) should be tested for H. pylori infection. Those who test positive should be offered treatment for the infection (strong recommendation, quality of evidence: high for active or history of PUD, low for MALT lymphoma, low for history of endoscopic resection of EGC). In patients with uninvestigated dyspepsia who are under the age of 60 years and without alarm features, non-endoscopic testing for H. pylori infection is a consideration. Those who test positive should be offered eradication therapy (conditional recommendation, quality of evidence: high for efficacy, low for the age threshold). When upper endoscopy is undertaken in patients with dyspepsia, gastric biopsies should be taken to evaluate for H. pylori infection. Infected patients should be offered eradication therapy (Strong recommendation, high quality of evidence). Patients with typical symptoms of gastroesophageal reflux disease (GERD) who do not have a history of PUD need not be tested for H. pylori infection. However, for those who are tested and found to be infected, treatment should be offered, acknowledging that effects on GERD symptoms are unpredictable (strong recommendation, high quality of evidence). In patients taking long-term low-dose aspirin, testing for H. pylori infection could be considered to reduce the risk of ulcer bleeding. Those who test positive should be offered eradication therapy (conditional recommendation, moderate quality of evidence). Patients initiating chronic treatment with a non-steroidal anti-inflammatory drug (NSAID) should be tested for H. pylori infection (strong recommendation, moderate quality of evidence). Those who test positive should be offered eradication therapy. The benefits of testing and treating H. pylori in patients already taking NSAIDs remains unclear (conditional recommendation, low quality of evidence). Patients with unexplained iron deficiency (ID) anemia despite an appropriate evaluation should be tested for H. pylori infection. Those who test positive should be offered eradication therapy (conditional recommendation, high quality of evidence). Adults with idiopathic thrombocytopenic purpura (ITP) should be tested for H. pylori infection. Those who test positive should be offered eradication therapy (conditional recommendation, very low quality of evidence). There is insufficient evidence to support routine testing and treating of H. pylori in asymptomatic individuals with a family history of gastric cancer or patients with lymphocytic gastritis, hyperplastic gastric polyps and hyperemesis gravidarum (no recommendation, very low quality of evidence). The ACG’s 2007 treatment guideline on the management of H. pylori infection (26) listed the following as established indications for diagnosis and treatment: Active PUD (gastric or duodenal). Confirmed history of PUD (not previously treated for H. pylori). Gastric MALT lymphoma (low grade). After endoscopic resection of EGC. The current guideline extends the list of potential indications to test patients for H. pylori infection. There are varying levels of evidence in support of the different potential indications for testing that are listed below. For some of these, the decision to test an individual patient for H. pylori will be influenced by clinical judgment and considerations of a patient’s general medical condition. Not all of these potential indications are given a definite recommendation, so that clinicians may exercise their judgment for individual patients. There is no justification in North America for universal or population-based screening. PUD The evidence in support of the 2007 recommendation was substantive at that time and these broad recommendations are still pertinent. All patients with a new diagnosis or a past history of PUD should be tested for H. pylori infection. Ideally, tests which identify active infection such as a urea breath test, fecal antigen test, or when endoscopy is performed, mucosal biopsy-based testing should be utilized. Because of the higher pretest probability of infection, patients with documented PUD represent a rare group, where it is acceptable to utilize an IgG H. pylori antibody test. In most other circumstances where the pretest probability of infection is lower, tests which identify active disease are preferred over antibody testing. Patients with a history of PUD who have previously been treated for H. pylori infection should undergo eradication testing with a urea breath test or fecal antigen test. Patients with evidence of ongoing infection should be treated appropriately. Gastric mucosa-associated lymphoid tissue (MALT) lymphoma The term “MALT lymphoma” has largely been supplanted by “marginal zone B-cell lymphoma of MALT type”. Identification of this neoplasm remains a key indication to test for, and to eradicate, H. pylori infection. A review published in 2009 identified and summarized six prospective cohort studies of treatment for H. pylori infection in patients with gastric MALT lymphoma (also referred to as “localized B-cell lymphoma of the stomach”) but found no systematic reviews or randomized controlled trials (27). Tumor regression was reported in 60–93% of patients after eradication of H. pylori infection, but response was inconsistent, with some patients showing a delayed response and some showing tumor relapse within a year of treatment. More recent studies have confirmed these observations. In a Japanese series, 77% out of 420 patients treated for H. pylori infection showed either complete histological response or probable minimal residual disease (the investigators’ definition of response), although 10 (3%) responders relapsed in a mean of 6.5 years (28). Among infected patients who did not respond to eradication treatment, there was progression of the disease in 27%. Among 120 patients in Germany followed for a median of 122 months, there was initial complete remission in 80% following treatment of H. pylori infection (29). Out of these, 3% had macroscopic recurrence of disease within 24 months, and another 17% had histological residual disease found after a median of 48 months. A recent review has suggested that treatment of H. pylori infection may also be beneficial for patients diagnosed with diffuse large B-cell lymphoma of the stomach (30). Early gastric cancer Three recent meta-analyses have each found that the incidence of metachronous gastric cancer following the endoscopic resection of a gastric neoplasm was reduced by the eradication of H. pylori infection (31, 32, 33). The most inclusive analysis by Yoon et al. (33) included 13 studies (three prospective and 10 retrospective) comprising 6687 patients. The pooled OR of gastric cancer in patients successfully cured of H. pylori was 0.42 (95% CI 0.32–0.56); in a subgroup analysis of the three prospective studies, the OR was 0.39 (95% CI 0.20–0.75) (33, 34). The other two meta-analyses yielded similar results (31, 32). Most recently, a meta-analysis comprising 24 studies (22 out of which were conducted in Asia) confirmed a lower rate of metachronous EGC following treatment of H. pylori infection; the incidence rate ratio was 0.54 (95% CI 0.46–0.65) (34). Dyspepsia (uninvestigated) Dyspepsia (defined as pain or discomfort centered in the upper abdomen) is highly prevalent in North America and elsewhere. In North America, most patients with dyspepsia will not have serious underlying, organic disease to explain their symptoms. That is, most will be found to have functional dyspepsia (FD), which is discussed elsewhere in this guideline. The ACG’s 2007 guideline on H. pylori management (26) included uninvestigated dyspepsia (depending upon H. pylori prevalence) in its list of established indications for diagnosis and treatment of H. pylori infection. The test and treat strategy for H. pylori infection was endorsed for patients under age 55 with dyspeptic symptoms and without alarm features. In the UK, the Bristol Helicobacter Project randomized 1517 H. pylori-positive adults to treatment for H. pylori infection or placebo and followed them prospectively (35). Among those treated for the infection, of whom over 90% achieved successful eradication, there was a small but statistically significant (P<0.05) reduction in subsequent consultations at the primary care level for dyspeptic complaints. The Cochrane Collaboration’s review on initial management strategies for dyspepsia was published in 2005 (36). As of early 2016, it had not been updated. A “test and treat” strategy for H. pylori had been found to be more effective than empirical acid suppression with either a proton pump inhibitor (PPI) or H2-receptor antagonist in managing dyspepsia (relative risk (RR) 0.59; 95% CI 0.42–0.83). This conclusion differs from an individual patient data meta-analysis which included three RCTs of 1537 patients randomized to the “test and treat” strategy or empirical acid suppression for the management of dyspepsia in the primary care setting (37). Although there was no significant difference between the groups in terms of symptom cure at 12 months, there was a trend for reduced overall costs in those assigned to “test and treat”. An individual patient data meta-analysis included five RCTs of 1924 patients randomized to “test and treat” or to prompt upper endoscopy for the evaluation of dyspeptic symptoms (38). After 1 year, the RR of remaining symptomatic was 0.95 (95% CI 0.92–0.99) in favor of prompt endoscopy. However, costs were lower with the “test and treat” approach. Prompt endoscopy for all patients with dyspepsia is neither feasible nor cost-effective. Functional dyspepsia A Cochrane systematic review published in 2006 concluded that there was a small but statistically significant benefit of treating H. pylori infection in patients with FD (39). In 17 RCTs comprising over 3500 patients, the RR reduction seen with treatment of H. pylori infection was 10% (95% CI 6–14%) and the number needed to treat (NNT) to cure one patient with FD was 14 (95% CI 10–25) (39). A subsequent update of that Cochrane review included 21 trials comprising 4331 patients (40). Most trials assessed patients’ symptoms 12 months after treatment. This study validated the NNT of 14 but with a narrower 95% CI (10–20). The Rome IV criteria have suggested subgrouping patients with FD into two groups, epigastric pain syndrome (epigastric pain and/or burning) or post-prandial distress syndrome (meal-related early satiation and/or fullness), while acknowledging that there may be considerable overlap between these (41). Although treatment trials have not utilized these newest criteria, improvement has been shown for patients with either predominant epigastric pain or predominant dysmotility-type symptoms following eradication of H. pylori infection (40). Since some FD patients with H. pylori infection experience durable benefit following eradication therapy, we recommend testing for, and treating, H. pylori in patients with FD. This aligns with a recent guideline by the American Gastroenterological Association, which recommends collecting biopsies of normal-appearing gastric mucosa to test for H. pylori when performing endoscopy in patients with dyspeptic symptoms (42). At the time of preparing this guideline, the ACG and the Canadian Association of Gastroenterology were in the process of preparing a joint guideline on management of uninvestigated dyspepsia and FD (43). Gastroesophageal reflux disease (GERD) There is no proven causal association between H. pylori infection and GERD. On a geographical basis, there is a negative association between the prevalence of H. pylori infection and the prevalence and severity of GERD (44). Barrett’s esophagus is more common among individuals who are not infected with H. pylori (45). The risk of esophageal adenocarcinoma among patients with Barrett’s esophagus is lower among those with H. pylori infection (45). The ACG’s 2007 guideline on H. pylori (26) reviewed the evidence for any change in GERD symptoms or severity following eradication of H. pylori infection. In North Americans who acquire H. pylori infection, the most likely phenotype is antral-predominant gastritis, hypergastrinemia, parietal cell hyperplasia, and increased gastric acid secretion. Such individuals who also have GERD may experience an improvement in GERD symptoms following eradication of H. pylori infection as gastric acid secretion slowly decreases in association with resolution of antral-predominant gastritis and hypergastrinemia (46). In a post hoc analysis of eight RCTs of the treatment of H. pylori infection in duodenal ulcer patients, there was no significant difference in the development of erosive esophagitis or GERD symptoms between those with successful and failed eradication (47). Among patients with pre-existing GERD, there was a worsening of symptoms in 7% of those cured of the infection and in 15% of those with persistent infection (OR=0.47; 95% CI 0.24–0.91; P=0.02). It is theoretically possible, however, that patients with corpus-predominant gastritis may experience onset or worsening of GERD symptoms following H. pylori eradication as a consequence of restitution of parietal cell mass and increased gastric acid secretion. However, this scenario should be relatively uncommon in North America. In a community-based study from the UK (the Bristol Helicobacter Project), treatment for H. pylori infection was not associated with an increase in the prevalence of heartburn or other reflux symptoms (48). Similarly, treatment for H. pylori infection did not improve reflux symptoms in patients with pre-existing symptoms. A systematic review of 27 published studies concluded that eradication of H. pylori infection from patients with duodenal ulcer did not predispose to the development of GERD (49) or worsen symptoms in patients with established GERD (49). Others have reported that cure of H. pylori infection in patients with erosive esophagitis before starting PPI therapy does not influence healing rates or symptom response (50, 51, 52). Therefore, based upon currently available evidence, there is no indication to test a patient with typical GERD symptoms for H. pylori infection unless that patient also has a history of PUD or dyspeptic symptoms. Patients with GERD who are tested for H. pylori infection for any reason and who are found to be positive should be offered treatment for the infection acknowledging that GERD symptoms are unlikely to improve. Long-term treatment with PPIs in H. pylori-positive individuals with corpus-predominant gastritis may promote the development of atrophic gastritis (53, 54). Although eradication of the infection before initiating PPI therapy may prevent the progression to atrophic gastritis (55), the clinical relevance of this is unclear. Low-dose aspirin use Aspirin (acetylsalicylic acid, ASA) is frequently recommended for patients with cardiovascular risk factors or following a major cardiovascular event (56). ASA use increases the risk of upper GI tract ulceration. H. pylori infection is a recognized risk factor for the development of ulcers and for ulcer bleeding during low-dose ASA treatment (57, 58). In a study conducted in Canada, Australia, the UK and Spain, the prevalence of peptic ulcer at endoscopy among 187 middle-aged and elderly patients taking ASA in doses of 75–325 mg daily was 10.7% (95% CI 6.3–15.1%) (59). H. pylori infection was a significant risk factor for ASA-related duodenal ulcer (OR=18.5; 95% CI 2.3–149.4) but not for gastric ulcer (OR=2.3; 95% CI 0.7–7.8). In a study from Hong Kong, patients with an episode of peptic ulcer bleeding while on low-dose ASA were studied according to H. pylori status (60). Those who were cured of H. pylori infection and subsequently re-started on ASA, had a similar rate of recurrent ulcer bleeding as in a previously ASA-naive cohort without bleeding who were started on low-dose ASA. Thus, eradication of H. pylori infection from patients with ASA-associated ulcer bleeding reduces the risk of recurrent bleeding. Regarding other anti-platelet agents, the 2010 expert consensus document jointly prepared by the ACG, the American College of Cardiology Foundation and the American Heart Association acknowledged that H. pylori infection was an established risk factor for upper GI bleeding among patients using thienopyridine anti-platelet agents (61) but made no specific recommendations concerning testing for, or treating, the infection in patients taking these medicines. However, testing for H. pylori will commonly be indicated in patients using these agents since most will also be taking ASA. In the absence of a prospective randomized study addressing H. pylori eradication in North American patients at increased risk for adverse cardiovascular outcomes, we suggest testing for H. pylori when starting prophylactic low-dose aspirin, while acknowledging that the evidence base for this recommendation is weak. Non-steroidal anti-inflammatory drug (NSAID) use H. pylori infection is an independent risk factor for NSAID-induced ulcers and ulcer bleeding (62, 63). Eradication of H. pylori infection before starting NSAID treatment reduces the development of ulcers and risk of ulcer bleeding (64, 65). A 2005 meta-analysis of five RCTs suggested that eradication of H. pylori infection among patients taking NSAIDs was associated with a 57% reduction in the incidence of peptic ulcer (OR=0.43; 95% CI 0.20–0.93) (66). The benefits of H. pylori eradication were greatest in patients who were previously NSAID-naive. Eradication of H. pylori infection before starting NSAID therapy may be the single most cost-effective strategy for the primary prevention of NSAID-associated ulcers in adult patients (67). The benefit of H. pylori eradication in patients already taking NSAIDs is less clear (68). RCTs suggest that H. pylori eradication does not reduce the incidence of new peptic ulcers in chronic NSAID users (69) and that PPI therapy provides a more effective ulcer risk reduction strategy than H. pylori eradication in patients on chronic NSAIDs (66). The ACG’s most recent practice guideline on the prevention of NSAID-related ulcer complications (63) concluded that H. pylori infection increases the risk of NSAID-related GI complications, that there would be at least a potential advantage of testing for the infection in patients requiring long-term NSAID therapy, and that the infection should be eradicated when identified. Iron deficiency anemia H. pylori infection has been associated with ID and iron deficiency anemia (IDA). In a meta-analysis of observational studies, the pooled ORs for ID and IDA in H. pylori-infected individuals were 1.4 (95% CI 1.2–1.6) and 2.0 (95% CI 1.5–2.9), respectively. A separate meta-analysis of 15 observational studies also found that IDA was more prevalent in H. pylori-infected individuals compared with H. pylori-negative controls (OR=2.2; 95% CI 1.5–3.2) (70). Adolescents with IDA have been reported to have a higher prevalence of H. pylori infection than non-anemic controls (71). H. pylori-infected adolescents and adults with IDA respond to oral iron therapy whether or not accompanied by treatment for H. pylori infection. However, the response to iron therapy in H. pylori-infected patients with IDA may be enhanced by the concomitant eradication of the infection (71, 72). A meta-analysis of 16 RCTs conducted among patients with IDA and H. pylori infection found statistically significant differences in favor of H. pylori eradication with oral iron over oral iron alone for increases in hemoglobin (Hgb), serum iron and serum ferritin (SF) levels (P<0.00001 for each) (73). In a separate meta-analysis of four interventional trials, the weighted mean difference in Hgb levels in favor of combined eradication treatment and oral iron vs. oral iron alone was 4.1 g/dl (95% CI −2.6–10.7); that for SF levels in five trials was 9.5 μg/l (95% CI −0.5–19.4) (70). A Cochrane systematic review is being conducted on the topic of the eradication of H. pylori infection for ID but its findings have not yet been reported (74). Idiopathic thrombocytopenic purpura (ITP) There is evidence from small randomized and non-randomized trials for a sustained improvement in platelet counts after eradication of H. pylori infection in a proportion of adult patients with ITP (75, 76, 77). The evidence is less compelling for children with ITP (78). A systematic review of 25 studies (1555 adult patients), all of which included at least 15 patients, found that platelet counts in ITP patients tended to increase after H. pylori eradication (79). Among 696 evaluable patients, 43% achieved a complete response (defined as a platelet count ≥100 × 109/l), and an additional 50% had an overall response (defined as a platelet count ≥30 × 109/l and at least a doubling of the baseline platelet count). Response rates were lower in patients with a baseline platelet count of <30 × 109/l. In general, response rates were higher in regions with high background H. pylori prevalence and in patients with mild degrees of thrombocytopenia. In their practice guideline published in 2011 (80), the American Society of Hematology (ASH) suggested that “screening for H. pylori infection be considered in adults with ITP in whom eradication therapy would be used if testing is positive” (evidence grade 2C). The ASH also recommended that eradication therapy be administered to adults with ITP who were found to have H. pylori based on a test of active infection (evidence grade 1B). The ASH has recommended against testing children with ITP for H. pylori infection (80). Asymptomatic individuals and the risk of gastric cancer Evidence that eradication of H. pylori infection reverses the gastric premalignant changes of gastric atrophy and intestinal metaplasia is conflicting. A meta-analysis of 12 studies (2658 patients) published up until 2009 reported that eradication was associated with a significant reduction in atrophic gastritis in the corpus (P=0.006) but not in the antrum (P=0.06); there was no evidence for a significant effect on intestinal metaplasia in either the corpus (P=0.42) or antrum (P=0.76) (81). A Cochrane systematic review and meta-analysis examined six RCTs (five of which were in Asian populations) that studied the effects of H. pylori eradication treatment against either placebo or no treatment on the subsequent development of gastric cancer among asymptomatic and otherwise healthy-infected adults (82). All trials followed subjects for at least 2 years. The quality of evidence was assessed as moderate. The subsequent incidence of gastric cancer was 1.6% among 3294 treated individuals and 2.4% among 3203 untreated controls (RR=0.66; 95% CI 0.46–0.95). The overall NNT was 124 (95% CI 78–843). However, assuming that the benefits of H. pylori eradication persist for life, the NNT could be as low as 15 among Chinese men. Since the lifetime risk of gastric cancer is lower in the United States, the corresponding NNT to prevent one case here would be 95 for men and 163 for women. A recent meta-analysis of 24 studies, 22 of which were conducted in Asia, showed that treatment of H. pylori infection in asymptomatic, infected adults led to a reduced incidence of gastric cancer. The greatest benefit was seen in people living in regions with the highest incidence of gastric cancer; reported RRs for regions of low, intermediate and high incidence of gastric cancer were, respectively, 0.80, 0.49, and 0.45 (34). Other gastrointestinal and non-gastrointestinal disorders Associations have been proposed between H. pylori infection and numerous other disorders (83). In most cases, biological plausibility and the level of evidence to support a causal association has been weak to non-existent. Thus, no formal recommendation can be offered. Controlled trials have suggested a benefit of H. pylori eradication in healing lymphocytic gastritis (84) and inducing regression of hyperplastic gastric polyps (85). There is some data to suggest a weak association between H. pylori infection and hyperammonemia and hepatic encephalopathy (HE) in patients with cirrhosis (86); trials evaluating H. pylori therapy in patients with HE have yielded conflicting results. In a meta-analysis of observational studies, the prevalence of H. pylori infection in pregnant women with hyperemesis gravidarum was higher than in matched controls (87). An association between H. pylori infection and major cardiovascular events including myocardial infarction and stroke has been postulated. However, evidence is of low quality and insufficient to establish causality (88). A Cochrane systematic review of H. pylori infection in Parkinson’s disease (89) identified three clinical trials and concluded that there was insufficient evidence to support screening for H. pylori infection in this population. Limited evidence suggests that symptoms of Parkinson’s may improve with H. pylori eradication, perhaps due to increases in the absorption and bioavailability of levodopa. Further randomized, controlled trials were encouraged. An evidence-based review of treating H. pylori infection in patients with urticaria found only low-quality evidence (90); nine out of 10 trials identified showed no benefit from H. pylori eradication. A meta-analysis has reported higher levels of glycosylated hemoglobin in H. pylori-positive patients with type 1 diabetes compared with non-infected patients (91). However, glycemic control did not improve in the short term following eradication of H. pylori. An inverse association has been reported between the prevalence of H. pylori infection and obesity (92). There may also be a weak inverse association between H. pylori infection and allergic or atopic disorders (93, 94) including eosinophilic esophagitis (95, 96, 97, 98, 99, 100) as well as celiac disease (101) and inflammatory bowel disease (102). Question 3: What Are Evidence-Based First-Line Treatment Strategies for Providers in North America? Recommendations Patients should be asked about any previous antibiotic exposure(s) and this information should be taken into consideration when choosing an H. pylori treatment regimen (conditional recommendation, moderate quality of evidence). Clarithromycin triple therapy consisting of a PPI, clarithromycin, and amoxicillin or metronidazole for 14 days remains a recommended treatment option in regions where H. pylori clarithromycin resistance is known to be <15% and in patients with no previous history of macrolide exposure for any reason (conditional recommendation, low quality of evidence (for duration: moderate quality of evidence)). Bismuth quadruple therapy consisting of a PPI, bismuth, tetracycline, and a nitroimidazole for 10–14 days is a recommended first-line treatment option. Bismuth quadruple therapy is particularly attractive in patients with any previous macrolide exposure or who are allergic to penicillin (strong recommendation, low quality of evidence). Concomitant therapy consisting of a PPI, clarithromycin, amoxicillin and a nitroimidazole for 10–14 days is a recommended first-line treatment option (Strong recommendation, low quality of evidence (for duration: very low quality of evidence)). Sequential therapy consisting of a PPI and amoxicillin for 5–7 days followed by a PPI, clarithromycin, and a nitroimidazole for 5–7 days is a suggested first-line treatment option (conditional recommendation, low quality of evidence (for duration: very low quality of evidence). Hybrid therapy consisting of a PPI and amoxicillin for 7 days followed by a PPI, amoxicillin, clarithromycin and a nitroimidazole for 7 days is a suggested first-line treatment option (conditional recommendation, low quality of evidence (For duration: very low quality of evidence)). Levofloxacin triple therapy consisting of a PPI, levofloxacin, and amoxicillin for 10–14 days is a suggested first-line treatment option (conditional recommendation, low quality of evidence (for duration: very low quality of evidence)). Fluoroquinolone sequential therapy consisting of a PPI and amoxicillin for 5–7 days followed by a PPI, fluoroquinolone, and nitroimidazole for 5–7 days is a suggested first-line treatment option (conditional recommendation, low quality of evidence (For duration: very low quality of evidence)). H. pylori is an infectious disease that is typically treated with combinations of 2–3 antibiotics along with a PPI, taken concomitantly or sequentially, for periods ranging from 3 to 14 days. In clinical practice, the initial course of eradication therapy, heretofore referred to as “first-line” therapy, generally offers the greatest likelihood of treatment success. Thus, careful attention to the selection of the most appropriate first-line eradication therapy for an individual patient is essential. There is no treatment regimen which guarantees cure of H. pylori infection in 100% of patients. Indeed, there are currently few, if any regimens which consistently achieve eradication rates exceeding 90% (103). In developing this guideline for North America, we conducted comprehensive literature searches to identify randomized, controlled trials which have evaluated the efficacy of treatment regiments in the United States and Canada. Wherever possible, we have tried to highlight these data and use them to develop treatment recommendations. Unfortunately, although H. pylori was the subject of many randomized, controlled trials conducted in North America during the first decade of this century, the number of treatment trials assessing modern regimens is modest to non-existent. As such, we were forced to rely upon clinical trial data generated in other parts of the world when considering a number of regimens. Development of this guideline has made clear the need for clinical trials to evaluate the efficacy of modern treatment regimens and organized efforts to monitor H. pylori antibiotic resistance in North America. To provide readers a sense of the author’s preferences, we have taken the liberty of utilizing the words “recommended” vs. “suggested” in the italicized statements that address each of the treatment regimens. A listing of available first-line treatment options can be found in Table 2. A schema to assist providers to choose the best therapy for an individual patient can be found in Figure 1. Table 2. Recommended first-line therapies for H pylori infection Regimen Drugs (doses) Dosing frequency Duration (days) FDA approval Clarithromycin triple PPI (standard or double dose) BID 14 Yesa Clarithromycin (500 mg) Amoxicillin (1 grm) or Metronidazole (500 mg TID) Bismuth quadruple PPI (standard dose) BID 10–14 Nob Bismuth subcitrate (120–300 mg) or subsalicylate (300 mg) QID Tetracycline (500 mg) QID Metronidazole (250–500 mg) QID (250) TID to QID (500) Concomitant PPI (standard dose) BID 10–14 No Clarithromycin (500 mg) Amoxicillin (1 grm) Nitroimidazole (500 mg)c Sequential PPI (standard dose)+Amoxicillin (1 grm) BID 5–7 No PPI, Clarithromycin (500 mg)+Nitroimidazole (500 mg)c BID 5–7 Hybrid PPI (standard dose)+Amox (1 grm) BID 7 No PPI, Amox, Clarithromycin (500 mg), Nitroimidazole (500 mg)c BID 7 Levofloxacin triple PPI (standard dose) BID 10–14 No Levofloxacin (500 mg) QD Amox (1 grm) BID Levofloxacin sequential PPI (standard or double dose)+Amox (1 grm) BID 5–7 No PPI, Amox, Levofloxacin (500 mg QD), Nitroimidazole (500 mg)c BID 5–7 LOAD Levofloxacin (250 mg) QD 7–10 No PPI (double dose) QD Nitazoxanide (500 mg) BID Doxycycline (100 mg) QD BID, twice daily; FDA, Food and Drug Administration; PPI, proton pump inhibitor; TID, three times daily; QD, once daily; QID, four times daily. a Several PPI, clarithromycin, and amoxicillin combinations have achieved FDA approval. PPI, clarithromycin and metronidazole is not an FDA-approved treatment regimen. b PPI, bismuth, tetracycline, and metronidazole prescribed separately is not an FDA-approved treatment regimen. However, Pylera, a combination product containing bismuth subcitrate, tetracycline, and metronidazole combined with a PPI for 10 days is an FDA-approved treatment regimen. c Metronidazole or tinidazole. Figure 1. Selection of a first-line H. pylori treatment regimen. With very few exceptions, the most common adverse events associated with antibiotics used to treat H. pylori infection are gastrointestinal in origin (for example: nausea, dysgeusia, dyspepsia/abdominal pain, diarrhea). As such, we have not listed adverse events for most of the therapies. Where unusual adverse events can occur with a specific therapy, we have tried to point that out. Clarithromycin triple therapy The previous ACG guideline from 2007 recommended 14 days of treatment with a PPI, clarithromycin, and amoxicillin (clarithromycin-based triple therapy) or—in patients with an allergy to penicillin—metronidazole as an alternative to amoxicillin. At that time, eradication rates for clarithromycin triple therapy were reported to be 70–85% and were highly influenced by the underlying rate of clarithromycin resistance (26). However, there has been growing concern regarding the efficacy of clarithromycin triple therapy. Key questions which were considered while preparing this document included the expected eradication rate of clarithromycin triple therapy in North America, the most appropriate duration of therapy, and whether eradication rates have been dropping over time. There are data from other parts of the world to suggest that eradication rates for clarithromycin triple therapy are below 80% (103). In preparing this updated guideline, we identified all randomized, controlled trials conducted in the United States or Canada which have assessed the efficacy of this regimen since 2000 (100, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119). Consistent with other meta-analyses, eradication rates with 7 or 10 days of clarithromycin triple therapy in studies from the US or Canada were indeed below 80%. Eradication rates with 14 days of triple therapy were higher, but only two study arms with 195 subjects were included. This finding is consistent with the most recent and most complete meta-analysis or the world’s literature on this topic which was published by the Cochrane Collaboration (120). For clarithromycin triple therapy, higher eradication rates were reported with 14 vs. 7 days of treatment (34 studies, RR 0.65, 95% CI 0.57–0.75; NNT 12, 95% CI 9–16), and with 14 vs. 10 days (10 studies, RR 0.69, 95% CI 0.52–0.91). Based upon the available data, when triple therapy is utilized in North America, it should be given for 14 days. The lack of recent RCT data on clarithromycin triple therapy makes it difficult to confidently report on temporal trends in eradication rates. To address this issue, we conducted a retrospective analysis of eradication rates for clarithromycin triple therapy at the University of Michigan from 2001–15. Data was divided into 5-year blocks and eradication rates for 10–14 days of triple therapy when given first-line were calculated. In 662 patients, the overall eradication rate was 79.5% (95% CI 77.2–82.4%) with no significant difference in eradication rates for the 3, 5-year blocks (Figure 2, unpublished data). Figure 2. Eradication rates with first-line clarithromycin triple therapy at the University of Michigan (2001–2015). The impact of clarithromycin resistance on the efficacy of clarithromycin triple therapy is well documented. A 2010 meta-analysis reported an eradication rate of 22% for clarithromycin-resistant H. pylori strains compared with 90% for clarithromycin-sensitive strains (121). As such, clarithromycin triple therapy should not be utilized in areas where the rate of clarithromycin resistance is known to be high. The Maastricht/Florence Consensus document published in 2012 recommended against using triple therapy when the rate of underlying clarithromycin resistance exceeds 15–20% (55). Although current large scale data on H. pylori antibiotic resistance in North America are unavailable, recent data from Houston suggest that clarithromycin resistance rates may now fall within that range (122). In the absence of local or even regional H. pylori antibiotic resistance data, it is very important to ask patients about previous exposure to antibiotics for any reason, particularly macrolides and fluoroquinolones, as this provides a proxy for underlying H. pylori antibiotic resistance (123, 124). A recent study confirmed an association between number of previous antibiotic exposures and an increasing risk for antibiotic resistance (125). Similarly, duration of previous macrolide therapy for greater than 2 weeks is also associated with a greater risk of treatment failure with clarithromycin triple therapy (126). Based upon the available data, we conclude that clarithromycin triple therapy consisting of a PPI, clarithromycin, and amoxicillin or metronidazole for 14 days remains a first-line treatment option in regions where H. pylori clarithromycin resistance is known to be low. In regions where clarithromycin resistance exceeds 15%, as may well be the case in many parts of North America, clarithromycin triple therapy should be avoided. All patients should be asked about previous macrolide exposure for any reason. In those with previous macrolide exposure, clarithromycin triple therapy should be avoided. Bismuth quadruple therapy The previous ACG guideline also endorsed the use of 10–14 days of bismuth quadruple therapy composed of a PPI or histamine-2 receptor antagonist, bismuth, metronidazole, and tetracycline. There is very limited data on the efficacy or comparative effectiveness of bismuth quadruple therapy in North America. A literature search identified only two RCTs which included a bismuth quadruple therapy arm (n=172). The mean eradication rate with this regimen given for 10 days was 91% (95% CI; 81–98%). A meta-analysis of studies from around the world comparing clarithromycin triple and bismuth quadruple therapies suggested that the two treatments had similar efficacy, compliance, and tolerability (121). An updated meta-analysis, which included 12 RCTs and 2753 patients, reported intention-to-treat (ITT) eradication rates of 77.6% with bismuth quadruple therapy vs. 68.9% with clarithromycin triple therapy (risk difference=0.06, 95% CI; −0.01 to 0.13). There was significant heterogeneity in the data set, in part attributable to differences in trea
Anaphylaxis is the most serious of all allergic reactions and can be fatal. The diagnosis is frequently delayed, and misdiagnosis often occurs with asthma or urticaria. Biomarkers such as tryptase are not routinely checked, and appropriate treatment with ...
Via Krishan Maggon

|
Scooped by
Gilbert C FAURE
April 7, 2018 6:37 AM
|
Acute urticaria may be associated with life-threatening angioedema and/or anaphylactic shock. Do you know key aspects of presentation, workup, and treatment? Test your knowledge with this short quiz.

|
Scooped by
Gilbert C FAURE
September 18, 2017 2:29 PM
|
Allergy Asthma Immunol Res. 2017 Nov;9(6):477-482. doi: 10.4168/aair.2017.9.6.477. Review

|
Scooped by
Gilbert C FAURE
January 26, 2017 8:35 AM
|
The introduction of omalizumab to the management of chronic spontaneous urticaria (CSU) has markedly improved the therapeutic possibilities for both, patients and physicians dealing with this disabling disease. But there is still a hard core of patients who do not tolerate or benefit from existing therapies and who require effective treatment. Novel approaches include the use of currently available drugs off-licence, investigational drugs currently undergoing clinical trials and exploring the potential for therapies directed at pathophysiological targets in CSU. Off-licence uses of currently available drugs include rituximab and tumour necrosis factor inhibitors. Ligelizumab (anti-IgE), canakinumab (anti-IL-1), AZD1981 (a PGD2 receptor antagonist) and GSK 2646264 (a selective Syk inhibitor) are currently in clinical trials for CSU. Examples of drugs that could target potential pathophysiological targets in CSU include substance P antagonists, designed ankyrin repeat proteins, C5a/C5a receptor inhibitors, anti-IL-4, anti-IL-5 and anti-IL-13 and drugs that target inhibitory mast cell receptors. Other mediators and receptors of likely pathogenic relevance should be explored in skin profiling and functional proof of concept studies. The exploration of novel therapeutic targets for their role and relevance in CSU should help to achieve a better understanding of its etiopathogenesis.
|


 Your new post is loading...
Your new post is loading...
 Your new post is loading...
Your new post is loading...


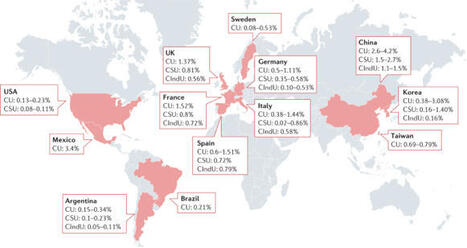


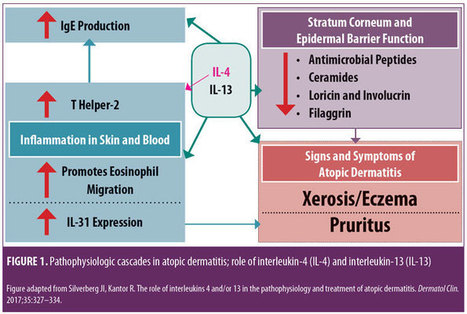
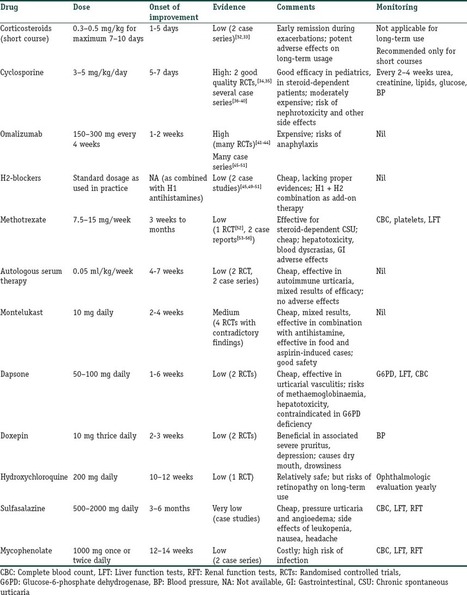





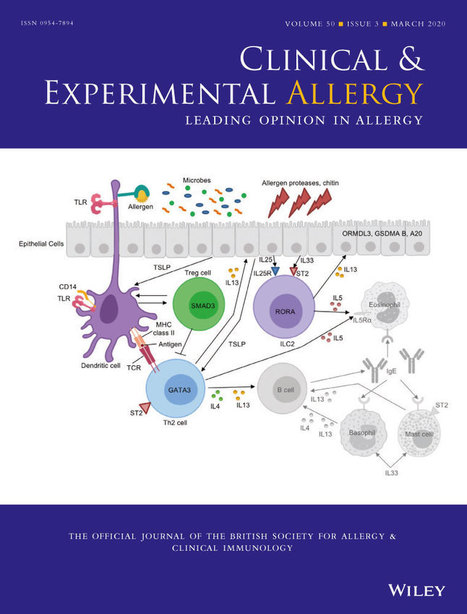


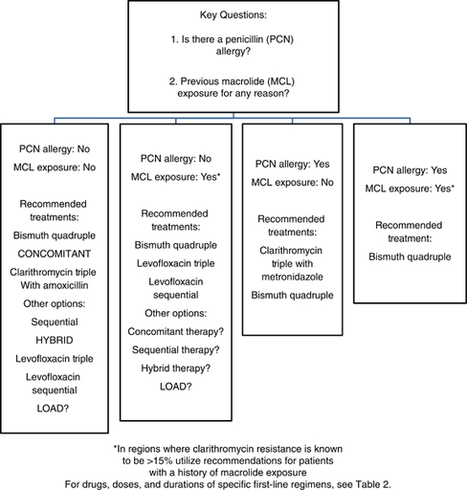
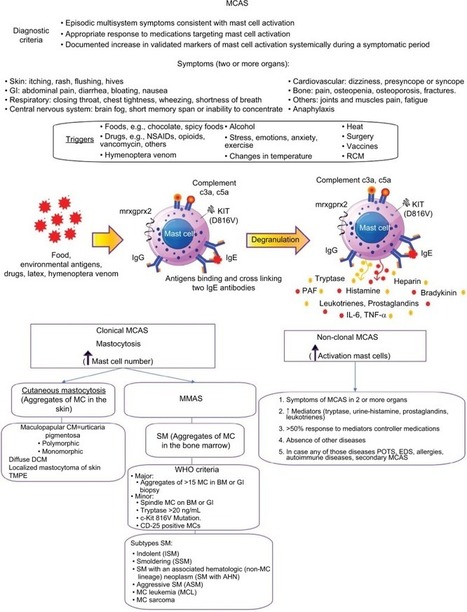







some links on https://www.scoop.it/topic/allergy-and-clinical-immunology?q=urticaria