Your new post is loading...
 Your new post is loading...

|
Scooped by
Gilbert C FAURE
March 9, 5:34 AM
|

|
Scooped by
Gilbert C FAURE
February 10, 6:32 AM
|

|
Scooped by
Gilbert C FAURE
April 23, 2022 11:06 AM
|
Research from King’s, led by Professor Gideon Lack and Dr Alexandra Santos, has made ground-breaking in-roads to our understanding of food allergy. Their work has changed the global understanding of peanut allergy and informed work on other food allergies, such as eggs, milk, shellfish and tree...

|
Scooped by
Gilbert C FAURE
January 26, 2022 8:18 AM
|
Peanut allergies affect 2% of children in western countries and are one of the most common causes of food related deaths in the UK.Standard care is dietary avoidance and access to self-injectable adrenaline (epinephrine), although a new treatment called Palforzia, containing peanut protein powder,...

|
Scooped by
Gilbert C FAURE
December 23, 2020 6:19 AM
|
FirstWord Pharma - Gain Access to the Information You Need Track the Companies,
Products, and Regulatory Areas of Most Interest to You

|
Scooped by
Gilbert C FAURE
May 9, 2020 4:43 AM
|

|
Scooped by
Gilbert C FAURE
March 5, 2020 2:12 PM
|
Some people produce immunoglobulin E (IgE) antibodies to proteins in common foods. As a result, these foods can trigger severe allergic inflammation (anaphylaxis). There are several structurally and functionally distinct antibody isotypes (IgM, IgD, IgG, IgA, and IgE), and which isotype binds to a target molecule (antigen) influences what happens next. For example, IgG that binds peanut proteins is harmless, but IgE bound to the same proteins can induce anaphylaxis and death. Therefore, how, where, and why allergen-reactive IgE is made are decades-old questions. Hoh et al. (1) found that gut tissue is a likely place for IgE development in peanut-allergic individuals. In addition, despite vast sequence possibilities, they found that many individuals share similar peanut-reactive IgE DNA sequences. This suggests that IgE antibodies in different individuals recognize peanut proteins in a similar manner, which could inform strategies for pharmacological interventions. Antibodies are produced by cells of the B lymphocyte lineage and consist of four Ig polypeptide chains—two identical heavy (H) chains and two identical light (L) chains—and each chain has a variable (V) region and a constant (C) region. The V region forms the surface that physically binds to antigens such as peanut proteins. The C region of IgH (CH) dictates antibody function largely by triggering processes that lead to neutralization, elimination, or induction of inflammation. Assembly of multiple IGH gene segments through the process of V(D)J recombination results in enormous sequence diversity, particularly in the exon encoding the V region, which comprises much of the antigen binding surface. Next to the V exon are a variety of C-region exons arranged in tandem (e.g., Cµ, Cγ, Cε, and Cα), which define the different antibody isotypes (2). Newly assembled Ig is produced as IgM. However, during an immune response, the same V region of IgH (VH) can be expressed in the context of another C-region isotype through a process called class switch recombination (CSR). This positions an alternative CH region next to the VH exon by permanent excision of the intervening DNA and associated CH-encoding exons. The position of CH exons relative to each other thus determines which IgH isotypes are available for secondary switch events. For example, an IgM-expressing B cell can switch to IgG1, and then that same IgG1+ B cell can switch to IgE because Cε is 3′ (downstream) to Cγ1 in the IGH locus. However, IgE-expressing B cells cannot switch to IgG1 (or any other isotype whose CH is positioned 5′ to Cε). Somatic hypermutation (SHM) further diversifies V regions by introducing mutations that can enhance affinity to antigen (3). The V(D)J recombination–, CSR-, and SHM-mediated processing of IGH enable phylogenetic mapping of how B-lineage cells and the antibodies they produce are related to one another. To gain insights into the origins of IgE production and the relationships between antibody-producing cells, Hoh et al. sequenced the IGH genes from B-lineage plasma cells in upper digestive tract tissues of 19 peanut-allergic individuals and compared them with those of nonallergic controls. They found more IgE-expressing cells in gut tissue in food-allergic individuals, confirming previous findings (4). Multiple clonally related VH-encoding sequences in IgE antibodies were also shared with other IgH isotypes. These findings suggest that B cells undergo CSR to IgE in the gut tissue as opposed to undergoing CSR to IgE elsewhere before migrating to the gut. This is different to the case of bone marrow, which is a major destination for antibody-producing cells after CSR elsewhere. This raises an important question: What features of the gut environment favor CSR to IgE? Moreover, because the bone marrow is a major location of antibody production, including IgE in allergic disease (5), the degree to which gut-derived versus bone marrow–derived IgE affects clinical disease, prognosis, and treatment approaches remains to be determined (see the figure). Hoh et al. identified antibody sequences that are reactive to the peanut protein Ara h 2 (Arachis hypogaea allergen 2) and found groups of similar sequences among multiple individuals. Similar sequences were also found in analyses of IgE+ B cells from peripheral blood in individuals with peanut allergy (6), further validating the concept of convergent IgE development to peanut proteins. Nonallergic individuals also had Ara h 2–reactive sequences, but only in non-IgE isotypes such as IgM, IgG, and IgA. These findings highlight how antibodies that induce a food-allergic response are generated. The production of antibodies that bind peanut proteins does not seem to be the problem per se; instead, the switching of that antibody to the IgE isotype appears to be key. This is consistent with observations that humans make IgG to a variety of dietary proteins without correlation to food allergy (7). In addition, it is possible that IgG to food antigens may be protective from food allergy by either blocking IgE binding or otherwise interfering with IgE function (8, 9). Perhaps an intervention that discourages gut IgE CSR could prevent food allergy. The convergence of IgE sequences in multiple peanut-allergic individuals suggests that immune recognition may occur through antibody binding to a few finite regions on key proteins. In this regard, drugs that block IgE binding to these regions holds promise as a therapy in allergic disease. There is proof of principle: Two therapeutic monoclonal IgG antibodies against a cat allergen inhibited IgE binding, and treatment with the combination of these two antibodies alone was sufficient to reduce allergic symptoms in 34 cat-allergic individuals in a clinical trial (10). Blocking IgE to peanut antigens may be similarly efficacious. Although seemingly innocuous, food allergens may influence the gut environment to generate conditions that induce CSR to IgE. In this regard, allergenic foods may have properties that induce allergic inflammation, as has been proposed (11, 12). Understanding the influences of the gut microbiota, age of exposure, and environment on the regulation of allergic responses to food (13, 14) promises to provide clues to elucidating how IgE CSR is regulated. http://www.sciencemag.org/about/science-licenses-journal-article-reuse This is an article distributed under the terms of the Science Journals Default License. References and Notes ↵ R. A. Hoh et al., Sci. Immunol. 5, eaay4209 (2020). ↵ P. Tong, D. R. Wesemann, Curr. Top. Microbiol. Immunol. 388, 21 (2015).OpenUrl ↵ L. Mesin, J. Ersching, G. D. Victora, Immunity 45, 471 (2016).OpenUrlPubMed ↵ C. Caffarelli, E. Romanini, P. Caruana, M. E. Street, G. De' Angelis, Pediatr. Res. 44, 485 (1998).OpenUrlPubMed ↵ S. Asrat et al., Sci. Immunol. 5, eaav8402 (2020). ↵ D. Croote, S. Darmanis, K. C. Nadeau, S. R. Quake, Science 362, 1306 (2018). ↵ J. Gocki, Z. Bartuzi, Postepy Dermatol. Alergol. 33, 253 (2016).OpenUrl ↵ O. T. Burton et al., J. Allergy Clin. Immunol. 134, 1310 (2014).OpenUrlCrossRef ↵ O. T. Burton, J. M. Tamayo, A. J. Stranks, K. J. Koleoglou, H. C. Oettgen, J. Allergy Clin. Immunol. 141, 189 (2018).OpenUrl ↵ J. M. Orengo et al., Nat. Commun. 9, 1421 (2018).OpenUrlCrossRef ↵ M. Profet, Q. Rev. Biol. 66, 23 (1991). ↵ N. W. Palm, R. K. Rosenstein, R. Medzhitov, Nature 484, 465 (2012). ↵ O. I. Iweala, C. R. Nagler, Annu. Rev. Immunol. 37, 377 (2019).OpenUrl ↵ D. R. Wesemann, C. R. Nagler, Immunity 44, 728 (2016).OpenUrl Acknowledgments: D.R.W. is supported by the NIH (AI121394, AI139538, and AI137940), the Burroughs Wellcome Fund, and an anonymous donor. C.R.N. is supported by the NIH (AI106302, AI134923, and AI146099) and the Sunshine Charitable Foundation. C.R.N. is president and co-founder of ClostraBio, Inc.

|
Rescooped by
Gilbert C FAURE
from Immunology and Biotherapies
January 14, 2020 4:37 AM
|
SAN FRANCISCO, CA / ACCESSWIRE / January 13, 2020 / Intrommune Therapeutics presented an update today on their oral mucosal immunotherapy (OMIT) technology at the Biotech Showcase™, which occurs during...
Via Krishan Maggon

|
Scooped by
Gilbert C FAURE
December 6, 2019 8:33 AM
|
Peanut allergies could become a thing of the past as breakthrough research from the University of South Australia develops a radically novel vaccination that’s poised to cure the potentially life threatening condition.

|
Rescooped by
Gilbert C FAURE
from Immunology and Biotherapies
October 30, 2019 3:51 PM
|
Peanut allergy is one of the most common and severe food allergies, affecting about
2% of school-aged children in the United Kingdom and United States. Current management of peanut allergy consists of strict allergen avoidance and carrying emergency medication to treat acute allergic reactions...
Via Krishan Maggon

|
Scooped by
Gilbert C FAURE
September 16, 2019 2:33 PM
|
Continuing with a modest dose confers more protection, NIH-funded study finds.

|
Suggested by
LIGHTING
July 23, 2019 9:00 AM
|
In daily practice, one-third of sesame allergic patients, confirmed by clinical history or food challenge, do not show any detectable specific IgE using current diagnostics. Currently used sesame extracts are water-based and therefore lacking hydrophobic proteins like oleosins. Oleosins, the stabilizer of lipid droplets in plants, are described as allergens in sesame, peanut and hazelnut. In this study, we examine the role of oleosins in sesame allergy and their potential cross-reactivity between sesame and (pea)nuts. Specific IgE and IgG sensitisation to native and heterologously expressed sesame components and oleosins from other nuts, free of seed storage proteins, was assessed by line blot and sera from 17 sesame allergic patients without detectable specific IgE sensitisation to sesame, and compared to 18 sesame allergic and 13 tolerant patients with specific IgE sensitisation to sesame. Sesame allergic patients without sensitisation showed no specific IgE to the tested sesame oleosins or components. Low levels of specific IgE to sesame oleosins were detected in 17% of sesame allergic and 15% of tolerant patients with sIgE sensitisation. Oleosins were recognised by serum IgG from multiple patients confirming immune reactivity and excluding technical issues leading to lack of specific IgE-binding to oleosins. Sesame oleosins are minor allergens and appear to have no additonal value in diagnosing sesame allergy in adults based on sIgE and sIgG detection. There is a high need for additional diagnostic tools in those patients to minimize the number of required food challenges.

|
Rescooped by
Gilbert C FAURE
from Immunology and Biotherapies
April 26, 2019 6:46 AM
|
In patients with peanut allergy, high-certainty evidence shows that available peanut
oral immunotherapy regimens considerably increase allergic and anaphylactic reactions
over avoidance or placebo, despite effectively inducing desensitisation.
Via Krishan Maggon
|

|
Scooped by
Gilbert C FAURE
February 15, 2:43 AM
|
FOOD ALLERGY NEWS: Eating gradually increasing doses of store-bought, home-measured peanut butter for about 18 months in a clinical trial enabled 100% of…

|
Scooped by
Gilbert C FAURE
October 5, 2024 7:06 AM
|
The prevalence of peanut allergy among children in Western countries has doubled in the past 10 years, and peanut allergy is becoming apparent in Africa and Asia. We evaluated strategies of peanut consumption and avoidance to determine which strategy ...

|
Scooped by
Gilbert C FAURE
February 16, 2022 9:25 AM
|
Original Article from The New England Journal of Medicine — Randomized Trial of Peanut Consumption in Infants at Risk for Peanut Allergy...

|
Scooped by
Gilbert C FAURE
January 21, 2021 6:02 AM
|
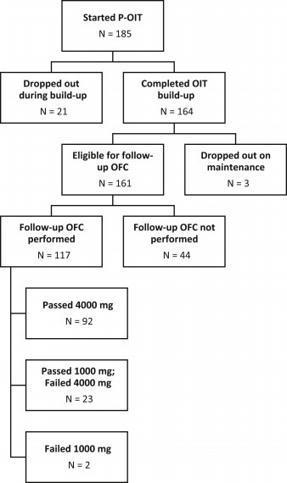
We previously described safety of preschool peanut oral immunotherapy (P-OIT) in a
real-world setting; 0.4% of patients experienced a severe reaction, and 4.1% received
epinephrine, during build-up.

|
Scooped by
Gilbert C FAURE
May 22, 2020 2:38 PM
|
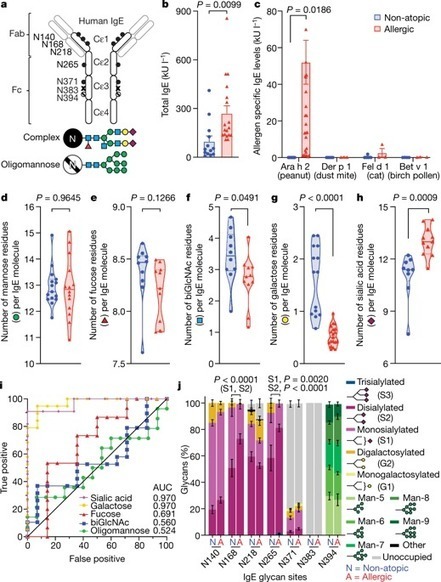
Approximately one-third of the world’s population suffers from allergies1. Exposure to allergens crosslinks immunoglobulin E (IgE) antibodies that are bound to mast cells and basophils, triggering the release of inflammatory mediators, including histamine2. Although IgE is absolutely required for allergies, it is not understood why total and allergen-specific IgE concentrations do not reproducibly correlate with allergic disease3–5. It is well-established that glycosylation of IgG dictates its effector function and has disease-specific patterns. However, whether IgE glycans differ in disease states or affect biological activity is completely unknown6. Here we perform an unbiased examination of glycosylation patterns of total IgE from individuals with a peanut allergy and from non-atopic individuals without allergies. Our analysis reveals an increase in sialic acid content on total IgE from individuals with a peanut allergy compared with non-atopic individuals. Removal of sialic acid from IgE attenuates effector-cell degranulation and anaphylaxis in several functional models of allergic disease. Therapeutic interventions—including removing sialic acid from cell-bound IgE with a neuraminidase enzyme targeted towards the IgE receptor FcεRI, and administering asialylated IgE—markedly reduce anaphylaxis. Together, these results establish IgE glycosylation, and specifically sialylation, as an important regulator of allergic disease. A specific type of glycosylation—sialylation—is more common on immunoglobulin E from individuals with a peanut allergys than from non-atopic people, suggesting that it has a role in regulating anaphylaxis.

|
Scooped by
Gilbert C FAURE
March 5, 2020 3:19 PM
|

|
Scooped by
Gilbert C FAURE
February 5, 2020 4:31 AM
|
The approval marks a “defining moment” for the peanut allergy community.- News - PharmaTimes...

|
Rescooped by
Gilbert C FAURE
from Immunology and Biotherapies
December 8, 2019 10:29 AM
|
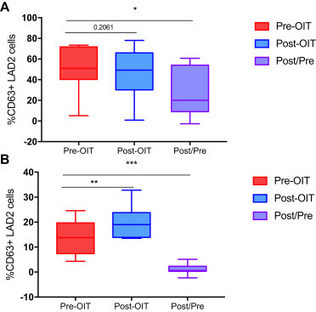
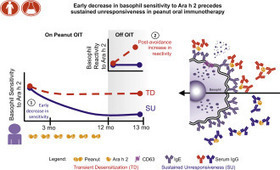
Only some patients with peanut allergy undergoing oral immunotherapy (OIT) achieve
sustained clinical response. Basophil activation could provide a functional surrogate
of efficacy.
Via Krishan Maggon

|
Scooped by
Gilbert C FAURE
November 17, 2019 5:41 AM
|
More than 3 million people in the United States are allergic to peanuts and tree nuts. But experimental new research suggests adults might someday be able to take an antibody to keep symptoms at bay for up to six weeks -- at least in small doses.

|
Rescooped by
Gilbert C FAURE
from Immunology and Biotherapies
October 12, 2019 2:43 AM
|
Abstract On July 10, 2019, the Institute for Clinical and Economic Review (ICER) published its final report on the effectiveness and value of AR101 and Viaskin® Peanut for peanut allergy1. At a thr...
Via Krishan Maggon

|
Rescooped by
Gilbert C FAURE
from Immunology and Biotherapies
September 15, 2019 1:22 PM
|
BRISBANE, Calif.--(BUSINESS WIRE)--Sep. 13, 2019-- Aimmune Therapeutics, Inc. (Nasdaq:AIMT), a biopharmaceutical company developing treatments for potentially life-threatening food allergies, today announced that the Allergenic Products Advisory Committee (APAC) convened by the U.S. Food and Drug Administration (FDA) voted to support the use of AR101 (proposed trade name PALFORZIA™) in children and teens with peanut allergy. PALFORZIA is a complex, biologic oral immunotherapy (OIT) candidate designed to reduce the incidence and severity of allergic reactions, including anaphylaxis, after accidental exposure to peanut in patients aged 4 through 17 years with a confirmed diagnosis of peanut allergy.
The APAC voted 7 to 2 that the efficacy data and 8 to 1 that the safety data, in conjunction with additional safeguards, are adequate to support the use of PALFORZIA.
Via Krishan Maggon

|
Scooped by
Gilbert C FAURE
July 18, 2019 5:05 AM
|
PubMed comprises more than 29 million citations for biomedical literature from MEDLINE, life science journals, and online books. Citations may include links to full-text content from PubMed Central and publisher web sites.
|

 Your new post is loading...
Your new post is loading...
 Your new post is loading...
Your new post is loading...