 Your new post is loading...
 Your new post is loading...
Allergen immunotherapy (AIT) treatment for allergic rhinitis and asthma is used by
2.6 million Americans annually. Clinical and sterility testing studies identify no
risk of contamination or infection from extracts prepared using recommended aseptic
techniques, but regulatory concerns persist.
Via Krishan Maggon

|
Scooped by
Gilbert C FAURE
July 23, 2019 2:13 PM
|
Asthma is a common lung disease affecting 300 million people worldwide. Allergic asthma is recognized as a prototypical Th2 disorder, orchestrated by an aberrant adaptive CD4+ T helper (Th2/Th17) cell immune response against airborne allergens, that leads to eosinophilic inflammation, reversible bronchoconstriction, and mucus overproduction. Other forms of asthma are controlled by an eosinophil-rich innate ILC2 response to epithelial damage, whereas in some patients with more neutrophilia, the disease is driven by Th17 cells. Dendritic cells (DCs) and macrophages are crucial regulators of type 2 immunity in asthma. Numerous lipid mediators including the eicosanoids prostaglandins and leukotrienes influence key functions of these cells, leading to either pro- or anti-inflammatory effects on disease outcome. In this review, we will discuss how eicosanoids affect the functions of DCs and macrophages in the asthmatic lung and how this leads to aberrant T cell differentiation that causes disease.

|
Scooped by
Gilbert C FAURE
June 21, 2019 11:41 AM
|
Asthma is prevalent but largely undiagnosed and undertreated in China. It is crucial
to increase the awareness of asthma and disseminate standardised treatment in clinical
settings to reduce the disease burden.

|
Scooped by
Gilbert C FAURE
June 2, 2019 4:36 AM
|
Summary Assessment and management of asthma is complicated by the heterogeneous pathophysiological mechanisms that underlie its clinical presentation, which are not necessarily reflected in standardized management paradigms and which necessitate an individualized approach to treatment. This is particularly important with the emerging availability of a variety of targeted forms of therapy that may only be appropriate for use in particular patient subgroups. The identification of biomarkers can potentially aid diagnosis and inform prognosis, help guide treatment decisions and allow clinicians to predict and monitor response to treatment. Biomarkers for asthma have been identified from a variety of sources, including airway, exhaled breath and blood. Biomarkers from exhaled breath include fractional exhaled nitric oxide, measurement of which can help identify patients most likely to benefit from inhaled corticosteroids and targeted anti‐immunoglobulin E therapy. Biomarkers measured in blood are relatively non‐invasive and technically more straightforward than those measured from exhaled breath or directly from the airway. The most well established of these are the blood eosinophil count and serum periostin, both of which have demonstrated utility in identifying patients most likely to benefit from targeted anti‐interleukin and anti‐immunoglobulin E therapies, and in monitoring subsequent treatment response. For example, serum periostin appears to be a biomarker for responsiveness to inhaled corticosteroid therapy and may help identify patients as suitable candidates for anti‐IL‐13 treatment. The use of biomarkers can therefore potentially help avoid unnecessary morbidity from high‐dose corticosteroid therapy and allow the most appropriate and cost‐effective use of targeted therapies. Ongoing clinical trials are helping to further elucidate the role of established biomarkers in routine clinical practice, and a range of other circulating novel potential biomarkers are currently being investigated in the research setting. Introduction Asthma is a heterogeneous disease that is usually characterized by chronic airway inflammation and structural change with associated airway dysfunction 1. It is defined by a history of respiratory symptoms (such as wheeze, shortness of breath, chest tightness and cough), which vary over time and in intensity, together with variable expiratory airflow limitation 1. Asthma affects approximately 300 million individuals worldwide, with prevalence rates ranging from 1% to 16% in different countries 2. In the UK, 5.4 million people currently receive treatment for asthma (1.1 million children [1 in 11] and 4.3 million adults [1 in 12]) and, on average, three people per day die from asthma 3. It is now clear that asthma comprises various disease subtypes with similar clinical manifestations, but with differing underlying pathophysiological mechanisms 1. The classification of asthma has consequently evolved as understanding regarding its pathophysiology has increased. Having initially been categorized in terms of ‘allergic’ or ‘non‐allergic’ asthma, a distinction was then made between ‘eosinophilic’ (‘type 2 high’) and ‘non‐eosinophilic’ (‘type 2 low’) asthma. Advances in disease understanding subsequently indicated that there may be subgroups of type 2 high asthma that differ in terms of both the presence of underlying allergy and the potential source of type 2 cytokines. This led to the current concept of ‘type 2 (T2) asthma’, which is characterized by high levels of type 2 interleukins (ILs), such as IL‐4, IL‐5 and IL‐13, and involves type 2 helper T cells (Th2 cells), mast cells, basophils, B cells and type 2 innate lymphoid cells (ILC2s) 4-6. It is currently believed that Th2 cells and ILC2s are primarily responsible for the production of the majority of type 2 cytokines in the airway, including IL‐4, IL‐5, IL‐9 and IL‐13 5, 7. The prolonged presence of activated inflammatory cells leads to chronic inflammation and airway remodelling 8. Since the 1990s, efforts to characterize asthma in terms of clinical, physiological and pathological parameters have led to the concept of asthma ‘phenotypes’ and ‘endotypes’ 9-13. The term ‘phenotype’ is used to denote a recognizable cluster of similar clinically observable characteristics, which can be identified using statistical methods (e.g. cluster analysis) without establishing an underlying aetiology or pathophysiology 12, 14, 15. By contrast, the term ‘endotype’ is used to describe a disease subtype with a clearly elucidated pathophysiology 15. Current approaches to asthma stratification primarily rely on identifying phenotypes, as the level of understanding required to establish endotypes has, in general, not yet been achieved 15. Asthma can be stratified in many ways: in terms of clinical history, physiology, inflammatory phenotype profile or biomarkers, and also in terms of therapeutic response to individual treatments. Stratification is central to the effective management of asthma, as it facilitates the personalization of treatment tailored to the individual's specific needs. This is particularly important with the emergence of targeted forms of asthma therapy, such as those targeting specific pro‐inflammatory cytokines 16. Asthma management in the UK is currently based on empirical stepwise treatment, with inhaled corticosteroids being standard of care for mild asthma, and combinations of inhaled corticosteroids and long‐acting beta agonist therapy plus further add‐on therapies, as required, for more severe asthma 17. International guidelines define severe asthma as ‘asthma that requires treatment with high‐dose inhaled corticosteroids plus a second controller and/or systemic corticosteroids to prevent it from becoming “uncontrolled,” or that remains ‘uncontrolled’ despite this therapy’ 18. Approximately 3–5% of asthma sufferers are unresponsive to available treatments and severe, therapy‐resistant asthma has become increasingly recognized as a major unmet need 18, 19. Moreover, severe refractory asthma is associated with a substantial burden in terms of healthcare costs. In the UK, the direct treatment costs from a National Health Service perspective, based on data from the British Thoracic Difficult Asthma Registry, were estimated to be £2912–4217 per patient per year 20. Identification of therapy resistance in difficult‐to‐treat asthma is hampered by poor adherence to treatment, which is known to be a problem in many asthma patients 21, 22. However, there is also a subset of patients who have poorly controlled asthma with persistent type 2 inflammation, despite adherence with high‐dose inhaled steroids. These patients often progress to systemic steroid treatment, which is associated with significant morbidity 23, and some of the new therapies targeting type 2 inflammation are likely to be of value for treating such patients in the future. It is therefore important to identify patient subgroups likely to benefit from targeted forms of treatment 19, 24. As the diagnosis of asthma is usually based on reported symptomatology and lung function tests 25, which are unable to assess airway inflammation and stratify the disease into discriminated phenotypes, there is a need for biomarkers to help with the accurate identification of clinically relevant phenotypes, not only to potentially aid diagnosis and inform prognosis, but also to guide treatment decisions, and predict and monitor treatment response. A biomarker is defined as ‘a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacological responses to a therapeutic intervention’ 26. The ideal characteristics of a biomarker are outlined in Table 1 15, 27-29. Essentially, the ideal biomarker should be easily measurable, have the ability to identify a mechanism known to be important in the pathogenesis of the disease, be reliable and reproducible in the clinical setting and be cost‐effective. It should ideally be mechanistically linked to the therapeutic target and responsive to intervention 15, 27-29. Biomarkers for asthma have been identified from a variety of sources, including the airway, exhaled breath and blood. The primary focus of this article is the role of T2 biomarkers in asthma management. Characteristic Details Easily measurable Non‐invasive Not requiring complex or potentially dangerous interventions Easy to collect in the ‘real‐world’ setting Ability to distinguish a mechanism causally linked to important clinical outcome High sensitivity, specificity, and positive and negative predictive values Correlation with treatment responses (e.g. to allow treatment adjustment) ’Normalization’ with successful treatment Reliable and reproducible in the clinical setting Little or no day‐to‐day variation (unless the variation is meaningful) Ability to provide information about disease prognosis and clinical outcomes Able to help inform disease management Mechanistically linked to the therapeutic target Able to provide better understanding of underlying pathophysiology Cost‐effective Airway biomarkers Bronchial biopsy has been considered the ‘gold standard’ for investigating airway inflammation and tissue remodelling, but it is invasive, costly, complex to perform and not readily accessible in clinics or research centres 28. Bronchoalveolar lavage also involves bronchoscopy, is often poorly tolerated by patients with asthma and also has to be conducted in a specialized hospital, preventing its widespread use in routine clinical practice 15. Sputum induction is less invasive and more cost‐effective than bronchoalveolar lavage, but it is still invasive and too technically complex and difficult to standardize for routine practice, and therefore generally only used in specialized centres and usually in a research setting 15, 25. Induced sputum and bronchoalveolar lavage contain cells (such as eosinophils and neutrophils) and supernatant containing cytokines, which can be used to predict asthma severity and exacerbations 25, 28. Indeed, the discernment of distinct inflammatory phenotypes through analysis of the cellular component of induced sputum is an early example of how biomarkers can be used to achieve asthma stratification 30, although the utility of these particular biomarkers in the personalization of treatment is unclear 15. Biomarkers in exhaled breath Fractional exhaled nitric oxide (FeNO) The measurement of FeNO is a relatively simple, fast, non‐invasive and reproducible technique that has been used as a surrogate measure of airway inflammation in asthma 15, 29. The American Thoracic Society and European Respiratory Society have standardized a method to measure FeNO 31, providing a simple, fast, non‐invasive and reproducible technique in asthma management 15. Findings from the Isle of Wight birth cohort study indicated that FeNO may be a useful biomarker for atopic asthma (where atopy was defined as having ≥ 1 positive skin prick test to either a food or aeroallergen) 32. High FeNO levels (> 47 ppb) have been shown to be associated with airway eosinophilia and corticosteroid responsiveness 33 and also to be prognostic for asthma exacerbations 34. Similarly, high (≥ 19.5 ppb) vs. low (< 19.5 ppb) FeNO levels have been associated with greater treatment effects with omalizumab, a targeted anti‐immunoglobulin E (IgE) therapy 35. Indeed, of the three potential biomarkers investigated in this study (FeNO, blood eosinophil count, serum periostin level), a high level of FeNO was the strongest predictor of response to omalizumab therapy 35. The degree of suppression of FeNO resulting from inhaled corticosteroid therapy has been used to identify non‐adherence to this treatment in a difficult‐to‐treat asthma population 36, and it has been suggested that this test could be used to help identify patients who are truly refractory to corticosteroid treatment, before considering dose escalation or the introduction of more costly targeted forms of therapy 22. In addition, a treatment algorithm in which FeNO concentrations were used to adjust patients’ inhaled corticosteroid dose during pregnancy was shown to significantly reduce the frequency of asthma exacerbations 37. However, the clinical utility of FeNO measurement in asthma is not clear‐cut, as studies investigating the association between asthma control and FeNO have yielded inconsistent results 25, perhaps partly because FeNO levels can be influenced by factors other than asthma, including age, medication use, smoking status and dietary factors 38. Controversy over the clinical utility of FeNO as biomarker for asthma is reflected in current treatment guidelines. In the UK, the National Institute of Health and Care Excellence Diagnostics Draft Guidance 12 (due for formal publication in 2017) advocates the use of FeNO testing to help diagnose asthma in adults and children 39; however, the Global Initiative for Asthma has concluded that FeNO cannot be recommended for asthma diagnosis and therapy monitoring 1. Volatile organic compounds (VOCs) VOCs can be measured in exhaled breath and their profile patterns might potentially be used to diagnose and distinguish between asthma and other conditions (e.g. chronic obstructive pulmonary disease), and discriminate between different asthma phenotypes 15, 40. ‘Electronic nose’ technology provides a means of studying VOCs in individual patients, by utilizing an array of sensors that react with different VOCs to generate a specific ‘breath print’ 41. This approach has demonstrated high sensitivity and specificity for discriminating between asthma, chronic obstructive pulmonary disease and healthy patients 42, and has been used in a clinical setting to discriminate between different inflammatory asthma phenotypes (eosinophilic, neutrophilic, paucigranulocytic) in patients with persistent asthma 40. It has also shown greater accuracy than measurement of FeNO or eosinophils for predicting responsiveness to corticosteroids 43. However, it does not appear to be able to distinguish mild asthma from severe asthma 44. The clinical utility of VOC measurement needs to be validated in a large asthma cohort; longitudinal data and guidelines for VOC measurement are also lacking 25. Exhaled breath condensate Exhaled breath condensate collection is an easy, non‐invasive, reproducible technique that can be used to measure several asthma biomarkers, including pH, markers of oxidative stress (including hydrogen peroxide), microRNA profiles, lipoxins, cytokines and leukotrienes 25, 28, 45. However, the technique is still in the research phase; a standardized methodology for exhaled breath condensate collection is required and reference values need to be established. Exhaled breath temperature Exhaled breath temperature is another potential biomarker for asthma, as blood flow in asthmatic airways is increased, resulting in a measurable increase in exhaled breath temperature 46, 47. Exhaled breath temperature is unlikely to be able to distinguish between asthma phenotypes, but it could potentially be used to monitor efficacy of asthma treatment, although this requires further research 15. Biomarkers in blood Biomarkers measured in blood are relatively non‐invasive, and less technically demanding than the assessment of biomarkers from exhaled breath. Several blood biomarkers are clinically well established and already used to help characterize asthma subtypes and monitor response to treatment. Certain novel blood biomarkers have also been identified, which may impact asthma management in the future. Eosinophil counts Peripheral blood eosinophil counts have been extensively studied as a potential biomarker for asthma. Eosinophils are important drivers of severe exacerbations in asthma 13, and blood eosinophil counts reflect inflammation in the asthmatic airway, being useful in the early detection of exacerbations and the regulation of steroid dosage 48. Mepolizumab, a humanized monoclonal antibody against IL‐5, is a selective and effective inhibitor of eosinophilic inflammation 49. Mepolizumab treatment has been shown to significantly decrease the frequency of exacerbations in patients with refractory asthma and evidence of eosinophilic airway inflammation, despite treatment with high doses of corticosteroids 13. These findings have been further supported by more recent trials of mepolizumab treatment in patients with eosinophilic asthma 50, 51. The benefits of treatment are closely associated with the blood eosinophil count, and the clinical response is marked in patients with pre‐treatment eosinophilia and absent in patients with a count < 150/μL 50. Similarly, Phase II trials of the anti‐IL‐5 agent, benralizumab, have demonstrated its effectiveness in reducing asthma exacerbation and improving lung function and asthma control in patients with uncontrolled eosinophilic asthma and acute asthma 52-54, and Phase III trials of the anti‐IL‐5 agent, reslizumab, have demonstrated its effectiveness in improving asthma control and symptoms, lung function and quality of life in patients with inadequately controlled asthma and elevated blood eosinophil counts 55-57. Blood eosinophil levels have also been shown to predict treatment benefit from biological therapies targeting IgE, IL‐4 and IL‐13 in patients with asthma 35, 58, 59. For example, in the EXTRA study, in which patients with uncontrolled severe persistent allergic asthma received 48 weeks of omalizumab treatment, patients with pre‐defined high baseline levels of peripheral blood eosinophil counts (≥ 260/μL) experienced a significant reduction in protocol‐defined exacerbations (–32%; P = 0.005), whereas those with pre‐defined low baseline levels (< 260/μL) did not (–9%; P = 0.54) 35. In a randomized, double‐blind, placebo‐controlled study of lebrikizumab (a monoclonal antibody to IL‐13), conducted in adults with asthma who were inadequately controlled despite inhaled glucocorticoid therapy, there was a trend towards a lower rate of protocol‐defined exacerbations in patients treated with lebrikizumab, compared with those treated with placebo, after 24 weeks (P = 0.16) 58. However, in the pre‐specified ‘Th2 high’ subgroup, defined on the basis of baseline peripheral blood eosinophil count and serum IgE level, the rate of exacerbations was 60% lower in the lebrikizumab group than in the placebo group (P = 0.03) 58. Although peripheral blood eosinophil data are readily available from a standard complete blood count, the clinical utility of the blood eosinophil count as a biomarker for asthma may be limited in some situations by its low specificity for eosinophilic airway inflammation, as a raised count can also be caused by other allergies, autoimmune disease and parasitic infections 25. In addition, the sensitivity of blood eosinophil levels as a biomarker for asthma is likely to decrease with the use of anti‐IL‐5 treatment, due to the differential effect of such treatment on eosinophilic inflammation in different tissue compartments. Consequently, a suppressed peripheral blood eosinophil count in patients receiving anti‐IL‐5 therapy does not reliably inform the level of underlying eosinophilic airway inflammation required to determine therapy response when symptoms and/or exacerbations remain persistent. Activation profile of eosinophils Changes in the activation profile of eosinophils in peripheral blood reflect their response to pro‐ and anti‐inflammatory signals; for example, membrane‐bound integrins become activated when eosinophils are primed to leave the circulation and enter tissue 60. Such changes have been used to diagnose the type and severity of allergic asthma 25. In a double‐blind, placebo‐controlled study of inhaled corticosteroid withdrawal in patients with mild asthma, activation of β1 integrins (identified using a monoclonal antibody) predicted decreased forced expiratory volume in 1 s more effectively than either FeNO or the percentage of eosinophils in sputum, and correlated with FeNO following inhaled corticosteroid withdrawal 61, 62. However, such differences in the activation profile of eosinophils are likely to be too subtle for routine application 25. Periostin Genomewide gene expression studies in bronchial epithelial cells identified two subsets of patients, ‘Th2 high’ and ‘Th2 low’ patients 63. This terminology was based on the observation that the highest expressed genes in the ‘Th2 high’ population were all inducible by IL‐13. Moreover, an analysis of gene expression in bronchial biopsies from the same patients demonstrated differences in IL‐13 gene expression levels between patients with ‘Th2‐high’ and ‘Th2‐low’ asthma or healthy controls 63. However, even with highly sensitive assays, differences in serum IL‐13 levels cannot reliably be detected 64. There is therefore a need for a surrogate systemic biomarker of Th2‐driven asthma (recently recognized as T2 asthma) that is mechanistically linked to IL‐13. Periostin, the gene for which was one of those most highly expressed in the Th2 high population in the study by Woodruff et al. 63, is induced by IL‐4 and IL‐13 in airway epithelial cells and lung fibroblasts 65. Periostin is a matricellular protein that has been identified as a component of subepithelial fibrosis in bronchial asthma 65, 66 and which has a potential role in eosinophilic airway inflammation 67 and regulation of mucus production 68. These mechanisms suggest that periostin is a potential mediator of asthma 69. Importantly, periostin has been shown to be a systemic biomarker of T2, IL‐13‐driven, corticosteroid‐responsive asthma 63, 66. In the BOBCAT study, conducted in patients with asthma who remained symptomatic despite maximal inhaled corticosteroid treatment, serum periostin was found to predict eosinophilic airway inflammation (defined as ≥ 22 tissue eosinophils/mm2 on bronchial biopsy or sputum eosinophilia ≥ 3%) better than FeNO levels, blood eosinophil counts or serum IgE levels (Fig. 1) 70. However, in another study, serum periostin was unable to distinguish eosinophilic asthma (defined as sputum eosinophils ≥ 3%) from non‐eosinophilic airway inflammation, whereas blood eosinophil count and FeNO level were able to do so 71. Serum periostin levels in patients with asthma have been shown to be significantly higher than those in healthy subjects, and to correlate positively with blood eosinophil counts, serum IgE and eosinophil cationic protein levels (P < 0.05) 72. High serum periostin levels have also been shown to predict asthmatic activity after reduction in inhaled corticosteroids in patients with apparently stable, well‐controlled asthma 73. Similarly, serum periostin appears to be a useful biomarker for the development of airflow limitation in patients with asthma on prolonged treatment with inhaled corticosteroids 74. These findings indicate that serum periostin may be a biomarker for responsiveness to inhaled corticosteroid therapy, which may therefore be useful in helping to identify patients as suitable candidates for alternative targeted forms of therapy 75. Serum periostin has also been investigated as a biomarker for predicting treatment response to targeted asthma therapies. For example, patients with asthma with high baseline levels of serum periostin reportedly benefit most from anti‐IL‐13 therapy with lebrikizumab and tralokinumab 58, 76, 77. Recently, topline results from 2 Phase III, randomized, double‐blind, placebo‐controlled studies evaluating the efficacy and safety of lebrikizumab in patients with severe asthma were released (LAVOLTA I and II) 78. LAVOLTA I met its primary endpoint by demonstrating a significant reduction in the rate of asthma exacerbations in patients with high levels of serum periostin or blood eosinophils. However, the exacerbation reduction results observed in LAVOLTA II did not attain statistical significance 78. The potential prognostic role of periostin as a biomarker to predict exacerbations and to help guide asthma therapy is being further investigated in ongoing clinical trials, such as the UK Refractory Asthma Stratification Programme (RASP‐UK; see ‘Implications of biomarkers of type 2 inflammation for current clinical practice’ section) 24. Current evidence suggests that periostin can help identify patients with T2 asthma, but additional biomarkers are likely to be required in order to further subdifferentiate this phenotype 15. The use of periostin as a biomarker in asthma management is currently limited by the need for well‐established and validated cut‐off values for high and low serum periostin levels, and thus the need for standardized measurement techniques. In addition, the specificity of periostin for asthma may be confounded by the presence of several factors, including comorbidities in which it is also known to be involved, such as metastatic cancer, renal injury, bone fracture and osteoporosis 79-81. Chitinase‐like protein YKL‐40 Serum levels of the chitinase‐like protein, YKL‐40, have been shown to be significantly elevated in patients with asthma vs. controls 82-84. However, reports regarding correlations between serum YKL‐40 levels and asthma severity or other asthma biomarker levels (eosinophils, total serum IgE, FeNO) have been inconsistent 70, 82-85. YKL‐40 polymorphisms have been shown to be associated with asthma, bronchial hyperresponsiveness and reduced lung function 86. It remains to be determined whether YKL‐40 is involved in the pathophysiology of asthma or is simply a marker of extracellular tissue remodelling 87, and its clinical utility in asthma management remains to be established. ILC2S Emerging evidence has indicated that activated ILC2s are capable of producing large amounts of inflammatory cytokines and may play a crucial role in T2 asthma 88. In a recent study conducted in 150 patients with mild‐to‐moderate asthma and 42 healthy controls, the percentage of ILC2s in peripheral blood was shown to correlate significantly with sputum eosinophil counts (P < 0.001), and to have a sensitivity of 67.7% and a specificity of 95.3% when used to distinguish eosinophilic from non‐eosinophilic patients with asthma 89. The percentage of ILC2s in peripheral blood therefore appears to be a surrogate marker of airway eosinophilic inflammation 89, although the clinical utility of this as a potential biomarker in asthma management is likely to be limited by difficulties in measuring ILC2 counts. IgE Serum levels of total and allergen‐specific IgE have been considered as potential biomarkers for study population characterization and for the assessment of effectiveness in intervention studies in asthma 90. However, in clinical trials of the anti‐IgE therapy, omalizumab, pre‐treatment levels of total IgE or antigen‐specific IgE were inconsistent in predicting response to treatment 91, 92. The clinical utility of measuring IgE serum levels may be limited by low specificity 25. Other circulating biomarkers Many other circulating molecules and proteins have been investigated as potential biomarkers for asthma (Table 2). Whilst some of these are related to clinically important features cross‐sectionally, we do not currently have evidence that they are prognostic or capable of predicting treatment responses. Potential biomarker Details ECP Component of eosinophil secondary granules, released during degranulation Increased serum ECP levels are indicative of systemic eosinophilic inflammation 93 Serum ECP levels are often increased in patients with asthma 94 and correlate well with airway inflammation 95 May have an advantage as a marker of activated eosinophils, although there is little evidence that ECP is more informative than blood eosinophil counts Serum ECP levels are affected by other factors, in particular smoking 95 Eotaxin (CCL11) Potent and selective chemoattractant for human eosinophils 93 Serum eotaxin levels correlate with ECP levels in asthma patients 96 Has been used to predict symptom severity during tapering of inhaled corticosteroid treatment 97 DPP‐4 Glycoprotein induced from bronchial epithelial cells by IL‐13 stimulation; highly expressed in bronchial epithelial cells of untreated asthma patients 98 In a Phase IIb study of tralokinumab therapy in severe uncontrolled asthma, patients with baseline serum DPP‐4 levels higher than the population median experienced improvements in FEV1 and asthma quality of life questionnaire scores 77 RANTES Chemokine at sites of allergic inflammation Serum levels significantly increased in asthma patients vs. controls; and in moderate and severe vs. mild asthma 99 Serum levels correlate positively with absolute eosinophil counts and total serum IgE, and negatively with FEV1 99 Osteopontin Plays a role in Th2‐mediated inflammation Serum levels shown to be elevated in patients with asthma vs. healthy controls, but do not appear to correlate with disease severity 100, 101 Thymus and activation‐regulated chemokine (TARC; CCL17) Thought to be involved in type 2‐mediated inflammation Serum levels shown to be significantly higher in patients with asthma vs. healthy controls and to correlate with eotaxin levels in patients with asthma 102 Serum levels are also increased in other allergic conditions; in particular, atopic dermatitis 103 Appetite‐modulating factors: visfatin and ghrelin Visfatin is an appetite‐modulating pro‐inflammatory adipokine and ghrelin primarily exerts anti‐inflammatory effects Serum levels of visfatin and ghrelin found to be significantly increased in patients with asthma vs. healthy controls 104 Other cytokines and growth factors IL‐3, IL‐18, fibroblast growth factor, hepatocyte growth factor and stem cell growth factor‐β shown to be significantly higher in patients with poorly controlled asthma vs. healthy controls 105 IL‐3 and IL‐18 levels found to be significantly higher in patients with poorly controlled asthma vs. those with well‐controlled asthma 105 Levels of IL‐18, fibroblast growth factor, hepatocyte growth factor and stem cell growth factor‐β shown to correlate positively with poor asthma control and negatively with quality of life scores 105 IL‐23 may be involved in modulating Th17 cells, which are thought to have a pathogenic role in T2 asthma 106 IL‐23 levels found to be significantly higher in children with asthma vs. healthy controls and to have a strong inverse relationship with FEV1 107 Acute‐phase proteins: α(2)‐macroglobulin, haptoglobin, ceruloplasmin and hemopexin Shown to be capable of discriminating between patients with asthma, patients with COPD and healthy controls, using two‐dimensional gel electrophoresis 108 Complement components C3 and C4 Serum levels of C3, but not C4, shown to be elevated in children with stable asthma, with a positive correlation between serum C3 and the severity of asthma 109 Serum levels of C3 and/or C4 found to be elevated in the majority of patients with intermittent atopic asthma vs. healthy controls 110 CRP Non‐specific marker of systemic inflammation that can be routinely measured in clinical practice 111 CRP levels shown to be increased in patients with asthma vs. controls 112 Correlations found between CRP levels and airway obstruction (FEV1/forced vital capacity ratio) 113 and the risk of severe asthma 114 However, CRP is highly non‐specific and has not shown consistent correlation with asthma control 111 Neutrophil chemotaxis velocity Neutrophils obtained from patients with asthma migrate significantly more slowly than those from patients with non‐asthmatic allergic rhinitis 115 Chemotaxis velocity of 1.55 μm/min may represent a threshold that can identify asthma with diagnostic sensitivity and specificity of 96% and 73%, respectively 115 COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; DPP‐4, dipeptidyl peptidase‐4; ECP, eosinophil cationic protein; FEV1, forced expiratory volume in 1 s; IgE, immunoglobulin E; IL, interleukin; RANTES, regulated upon activation, normal T‐cell expressed and secreted; Th2, type 2 helper T cell; T2, type 2. Implications of biomarkers of type 2 inflammation for current clinical practice The management of severe asthma remains a daily challenge as we currently have no gold standard diagnostic test, easy measure of adherence to prescribed therapies or accurate predictor of future risk. Failure to respond to high‐dose inhaled corticosteroids in difficult‐to‐control asthma may either be due to non‐adherence to treatment (which occurs in 30–50% of patients) or may be due to persistent type 2 inflammation, which is relatively resistant to corticosteroid therapy. This latter group of patients requires additional treatment with agents that specifically target type 2 mechanisms (such as anti‐IL‐5 and anti‐IL‐13 treatments) 24. Conversely, patients with severe asthma frequently suffer from multiple comorbidities and may continue to be symptomatic even when their underlying airway inflammation is well controlled. RASP‐UK is currently conducting an objective assessment of corticosteroid adherence in patients with severe asthma and investigating the use of novel biomarker stratification strategies to improve clinical management 24. Specifically, the study will identify patients with high levels of T2 asthma biomarkers (periostin, eosinophils, FeNO) when adherent to high‐dose corticosteroid therapy as appropriate candidates for novel biological treatments that target the T2 cytokine axis 24. It is hoped that such stratification will enable patients to receive the most appropriate treatment, thus optimizing the use of relatively expensive targeted therapies. RASP‐UK will also compare the use of a composite biomarker score, comprising FeNO, serum periostin and blood eosinophils, to guide corticosteroid therapy vs. conventional symptom‐based guidelines, thereby potentially helping to avoid unnecessary morbidity due to excessive corticosteroid exposure 24. U‐BIOPRED (Unbiased BIOmarkers in PREDiction of respiratory disease outcomes) is a 5‐year European‐wide project that aims to identify biomarkers for severe asthma 116. It will make use of multi‐dimensional phenotyping using a series of ‘omics platforms’, in which a large number of parameters will be assessed at one time, allowing researchers to develop a ‘handprint’ of severe asthma subtypes 116. It is hoped that the project will help refine diagnostic criteria for asthma phenotypes and establish whether these can predict responsiveness to new and existing treatments 116, thereby providing a template for asthma stratification and personalization of treatment. Hopefully, these and other ongoing clinical studies will allow the use of blood biomarkers in asthma to move from the research setting to routine clinical practice. The emergence of a multiplicity of novel biomarkers for asthma, including those developed as companion diagnostics for targeted therapies, could lead to confusion and over‐complexity in clinical practice. It is hoped that studies such as RASP‐UK and U‐BIOPRED will provide the strength of data required to enable the development of evidence‐based guidelines detailing how and when specific biomarkers should be used, in terms of guiding prognosis, informing treatment decisions and monitoring subsequent response to therapy. To date, research has predominantly focussed on the characterization of biomarkers for T2 asthma. However, T2 asthma represents only one of the currently recognized asthma phenotypes. Non‐eosinophilic airway inflammation occurs in approximately 50% of patients with asthma, and non‐eosinophilic asthma has been further subdivided into neutrophilic, mixed granulocytic and pauci‐granulocytic (or pauci‐immune) subtypes 30, 117, 118. The proportions of these subtypes are currently unclear, due to variations in the cut‐offs used to define them 117. For example, non‐eosinophilic asthma has been defined as asthma associated with a sputum eosinophil count of either ≤ 2% or ≤ 3%, whilst neutrophilic asthma has been defined using sputum neutrophil cut‐offs ranging from ≥ 60% to > 76% 119. Crucially, non‐eosinophilic asthma is insensitive to corticosteroid therapy 9. To date, biological agents developed to target mediators of non‐eosinophilic inflammation (e.g. IL‐17) and novel small molecules targeting neutrophilic inflammation (e.g. chemokine receptor 2 antagonists) have failed to show convincing beneficial effects in clinical trials 117, 120, 121. There is therefore an ongoing need for research to further identify and characterize biomarkers of non‐T2 asthma, to aid diagnosis, inform prognosis and help guide the development of effective targeted therapies. The characterization of non‐T2 biomarkers is also important for the characterization and management of T2 asthma, as the absence of expression of a T2 biomarker does not necessarily imply a non‐T2 status, and biomarkers for non‐T2 asthma will therefore aid the differential diagnosis of both non‐T2 and T2 phenotypes. Conclusions The clinical management of airway diseases is not straightforward and is confounded by the use of diagnostic labels implying a probable natural history, which can result in suboptimal management paradigms and unhelpfully influence expectations about treatment outcomes 122, 123. Asthma is now known to be heterogeneous with a number of underlying pathophysiological mechanisms, which may require very different treatment approaches. Consequently, there has been an increasing focus on identifying specific, well‐defined and treatable aspects of disease 123, 124. The identification and evidence‐based clinical application of reliable biomarkers that: (1) assist in the diagnosis of asthma, (2) provide prognostic information and (3) identify underlying pathophysiological disease mechanism(s) to target treatment will be major areas of advance in coming years. Several biomarkers are currently available that assist in these areas (Table 3) but ongoing research will help clarify further the potential use of these and other biomarkers in routine practice, and provide greater understanding and evidence for the clinical utility of a range of emerging novel potential biomarkers. Biomarker Ideal characteristics of biomarker Easily measurable Ability to distinguish a mechanism causally linked to important clinical outcome Reliable and reproducible in the clinical setting Ability to provide information about disease prognosis and clinical outcomes Mechanistically linked to the therapeutic target Cost‐effective Bronchial biopsy − ++ − − ++ − Bronchoalveolar lavage − ++ − − ++ − Sputum induction + ++ − +++ + + FeNO ++ ++ +++ ++ ++ ++ VOCs ++ +++ + − ++ ? Exhaled breath condensate +++ + − ++ ++ ? Exhaled breath temperature +++ − − + + +++ Blood eosinophil counts +++ +++ +++ ++ +++ +++ Activation profile of eosinophils + ++ − − +++ ? Serum periostin +++ +++ +++ +++ +++ +++ Chitinase‐like protein YKL‐40 + − − − + ? Blood ILC2s − +++ − ++ ++ ? Serum IgE +++ − − + + ? FeNO, fractional exhaled nitric oxide; IgE, immunoglobulin E; ILC2s, type 2 innate lymphoid cells; VOCs, volatile organic compounds. Acknowledgements Third‐party medical writing assistance for this review paper was provided by John Scopes, mXm Medical Communications, and was funded by Roche Products Ltd. Author contributions All authors were involved in the analysis and interpretation of data included in this article, in the writing of the article and in the decision to submit the article for publication. All authors have reviewed and approved the final version for publication. Conflict of interests In the last 5 years, IDP has received speaker's honoraria from AstraZeneca, Boehringer Ingelheim, Aerocrine, Almirall, Novartis and GlaxoSmithKline, and a payment for organizing an educational event from AstraZeneca. He has also received honoraria for attending advisory panels with Almirall, Genentech, Regeneron, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Merck Sharp & Dohme, Schering‐Plough, Novartis, Dey, Napp and Respivert, and sponsorship to attend international scientific meetings from Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca and Napp. SA is an employee of Roche Products Ltd. AMG has attended advisory boards for Roche, AstraZeneca, Teva and Novartis; has received lecture fees from Roche, AstraZeneca, Teva, Novartis, Chiesi, Boehringer Ingelheim and Napp; has attended international conferences with Napp and Boehringer Ingelheim; and has participated in clinical trials with GlaxoSmithKline, Roche and Boehringer Ingelheim, for which his institution has been remunerated. LGH has received grant funding from MedImmune, Novartis UK, Hoffmann‐La Roche/Genentech Inc., AstraZeneca and GlaxoSmithKline; has taken part in advisory boards and given lectures at meetings supported by GlaxoSmithKline, Respivert, Merck Sharp & Dohme, Nycomed, Boehringer Ingelheim, Vectura, Novartis and AstraZeneca; has received funding support to attend international respiratory meetings (AstraZeneca, Chiesi, Novartis, Boehringer Ingelheim and GlaxoSmithKline); and has taken part in asthma clinical trials (GlaxoSmithKline, Schering‐Plough, Synairgen and Hoffmann‐La Roche/Genentech), for which his institution was remunerated. He is also Academic Lead for the Medical Research Council Stratified Medicine UK Consortium in Severe Asthma, which involves industrial partnerships with Amgen, Genentech/Hoffman‐La Roche, AstraZeneca, Medimmune, Aerocrine and Vitalograph. References Citing Literature Number of times cited according to CrossRef: 10 G. Roberts, Asthma and oral immunotherapy biomarkers, Clinical & Experimental Allergy, 49, 2, (140-141), (2019). Wiley Online Library Hitasha Rupani and Anoop J Chauhan, Measurement of FeNO in asthma: what the hospital doctor needs to know, British Journal of Hospital Medicine, 10.12968/hmed.2019.80.2.99, 80, 2, (99-104), (2019). Crossref Ian D. Pavord and Nicola A. Hanania, Controversies in Allergy: Should Severe Asthma with Eosinophilic Phenotype Always Be Treated with Anti-IL-5 Therapies, The Journal of Allergy and Clinical Immunology: In Practice, 10.1016/j.jaip.2019.03.010, (2019). Crossref Sun-Hye Lee, Pureun-Haneul Lee, Byeong-Gon Kim, Jisu Hong and An-Soo Jang, Annexin A5 Protein as a Potential Biomarker for the Diagnosis of Asthma, Lung, 10.1007/s00408-018-0159-x, 196, 6, (681-689), (2018). Crossref S. Diver, R. J. Russell and C. E. Brightling, New and emerging drug treatments for severe asthma, Clinical & Experimental Allergy, 48, 3, (241-252), (2018). Wiley Online Library Yunus Çolak, Shoaib Afzal, Børge G. Nordestgaard, Jacob L. Marott and Peter Lange, Combined value of exhaled nitric oxide and blood eosinophils in chronic airway disease: the Copenhagen General Population Study, European Respiratory Journal, 10.1183/13993003.00616-2018, 52, 2, (1800616), (2018). Crossref Lisa Giovannini-Chami, Agnès Paquet, Céline Sanfiorenzo, Nicolas Pons, Julie Cazareth, Virginie Magnone, Kévin Lebrigand, Benoit Chevalier, Ambre Vallauri, Valérie Julia, Charles-Hugo Marquette, Brice Marcet, Sylvie Leroy and Pascal Barbry, The “one airway, one disease” concept in light of Th2 inflammation, European Respiratory Journal, 10.1183/13993003.00437-2018, 52, 4, (1800437), (2018). Crossref Reynold A Panettieri, Ulf Sjöbring, AnnaMaria Péterffy, Peter Wessman, Karin Bowen, Edward Piper, Gene Colice and Christopher E Brightling, Tralokinumab for severe, uncontrolled asthma (STRATOS 1 and STRATOS 2): two randomised, double-blind, placebo-controlled, phase 3 clinical trials, The Lancet Respiratory Medicine, 10.1016/S2213-2600(18)30184-X, 6, 7, (511-525), (2018). Crossref Joo-Hee Kim, Serum vascular endothelial growth factor as a marker of asthma exacerbation, The Korean Journal of Internal Medicine, 32, 2, (258), (2017). Crossref Giovanni Passalacqua, Anti-interleukin 5 therapies in severe asthma, The Lancet Respiratory Medicine, 5, 7, (537), (2017). Crossref

|
Scooped by
Gilbert C FAURE
May 11, 2019 1:51 PM
|

|
Scooped by
Gilbert C FAURE
April 30, 2019 8:20 AM
|
Many children with asthma think they are using their asthma inhaler medications correctly when they are not. This makes it very difficult to keep their asthma under control. A new study in Annals of Allergy, Asthma and Immunology, ...

|
Suggested by
Société Francaise d'Immunologie
April 28, 2019 7:06 AM
|
Original Article from The New England Journal of Medicine — Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children...

|
Suggested by
LIGHTING
April 11, 2019 11:52 PM
|
Respiratory infections are the principal cause of asthma exacerbations in children. Altman and colleagues use a systems approach to describe the pathways associated with asthma exacerbations in a cohort of inner-city children.

|
Scooped by
Gilbert C FAURE
March 11, 2019 10:33 AM
|

|
Suggested by
Société Francaise d'Immunologie
March 6, 2019 4:56 AM
|
The neuronal and immune systems exhibit bidirectional interactions that play a critical role in tissue homeostasis, infection, and inflammation. Neuron-derived neuropeptides and neurotransmitters regulate immune cell functions, whereas inflammatory mediators produced by immune cells enhance neuronal activation. In recent years, accumulating evidence suggests that peripheral neurons and immune cells are colocalized and affect each other in local tissues. A variety of cytokines, inflammatory mediators, neuropeptides, and neurotransmitters appear to facilitate this crosstalk and positive-feedback loops between multiple types of immune cells and the central, peripheral, sympathetic, parasympathetic, and enteric nervous systems. In this Review, we discuss these recent findings regarding neuro-immune crosstalk that are uncovering molecular mechanisms that regulate inflammation. Finally, neuro-immune crosstalk has a key role in the pathophysiology of allergic diseases, and we present evidence indicating that neuro-immune interactions regulate asthma pathophysiology through both direct and indirect mechanisms.

|
Scooped by
Gilbert C FAURE
February 27, 2019 9:31 AM
|
Review Series 10.1172/JCI124610 Influences on allergic mechanisms through gut, lung, and skin microbiome exposures Andrea M. Kemter and Cathryn R. Nagler First published February 25, 2019 - More info In industrialized societies the incidence of allergic diseases like atopic dermatitis, food allergies, and asthma has risen alarmingly over the last few decades. This increase has been attributed, in part, to lifestyle changes that alter the composition and function of the microbes that colonize the skin and mucosal surfaces. Strategies that reverse these changes to establish and maintain a healthy microbiome show promise for the prevention and treatment of allergic disease. In this Review, we will discuss evidence from preclinical and clinical studies that gives insights into how the microbiota of skin, intestinal tract, and airways influence immune responses in the context of allergic sensitization. PREVIEW PAGES Reset NextPage 0Back Continue reading with a subscription. A subscription is required for you to read this article in full. If you are a subscriber, you may sign in to continue reading. Already subscribed? Click here to sign into your account. Don't have a subscription? Please select one of the subscription options, which includes a low-cost option just for this article. At an institution or library? If you are at an institution or library and believe you should have access, please check with your librarian or administrator (more information). Problems? Please try these troubleshooting tips.

|
Scooped by
Gilbert C FAURE
February 14, 2019 5:31 AM
|
Environmental exposures interplay with human host factors to promote the development and progression of allergic diseases. The worldwide prevalence of allergic disease is rising as a result of complex gene-environment interactions that shape the immune system and host response. Research shows an association between the rise of allergic diseases and increasingly modern Westernized lifestyles, which are characterized by increased urbanization, time spent indoors, and antibiotic usage. These environmental changes result in increased exposure to air and traffic pollution, fungi, infectious agents, tobacco smoke, and other early-life and lifelong risk factors for the development and exacerbation of asthma and allergic diseases. It is increasingly recognized that the timing, load, and route of allergen exposure affect allergic disease phenotypes and development. Still, our ability to prevent allergic diseases is hindered by gaps in understanding of the underlying mechanisms and interaction of environmental, viral, and allergen exposures with immune pathways that impact disease development. This Review highlights epidemiologic and mechanistic evidence linking environmental exposures to the development and exacerbation of allergic airway responses.
More and more clinical trials have tried to assess the clinical benefit of anti-interleukin (IL)-13 monoclonal antibodies for uncontrolled asthma. The aim of this study is to evaluate the efficacy and safety of anti-IL-13 monoclonal antibodies for uncontrolled asthma. Major databases were searched for randomized controlled trials comparing the anti-IL-13 treatment and a placebo in uncontrolled asthma. Outcomes, including asthma exacerbation rate, forced expiratory volume in 1 second (FEV1), Asthma Quality of Life Questionnaire (AQLQ) scores, rescue medication use, and adverse events were extracted from included studies for systematic review and meta-analysis. Five studies involving 3476 patients and two anti-IL-13 antibodies (lebrikizumab and tralokinumab) were included in this meta-analysis. Compared to the placebo, anti-IL-13 treatments were associated with significant improvement in asthma exacerbation, FEV1 and AQLQ scores, and reduction in rescue medication use. Adverse events and serious adverse events were similar between two groups. Subgroup analysis showed patients with high periostin level had a lower risk of asthma exacerbation after receiving anti-IL-13 treatment. Our study suggests that anti-IL-13 monoclonal antibodies could improve the management of uncontrolled asthma. Periostin may be a good biomarker to detect the specific subgroup who could get better response to anti-IL-13 treatments. In view of blocking IL-13 alone is possibly not enough to achieve asthma control because of the overlapping pathophysiological roles of IL-13/IL-4 in inflammatory pathways, combined blocking of IL-13 and IL-4 with monoclonal antibodies may be more encouraging.
Via Krishan Maggon
|
There are no currently available validated biomarkers that can predict AIT success. In adolescents and adults, AIT should be reserved for patients with moderate/severe rhinitis or for those with moderate asthma who, despite appropriate pharmacotherapy and adherence, continue to exhibit exacerbations that appear to be related to allergen exposure, except in some specific cases. Immunotherapy may be even more advantageous in patients with multimorbidity. In children, AIT may prevent asthma onset in patients with rhinitis. mHealth tools are promising for the stratification and follow‐up of patients.
Via Krishan Maggon

|
Scooped by
Gilbert C FAURE
July 15, 2019 2:29 AM
|
Allergic diseases, such as respiratory, cutaneous and food allergy, have dramatically increased in prevalence over the last few decades. Recent research points to a central role of the microbiome, which is highly influenced by multiple environmental and dietary factors. It is well established that the microbiome can modulate the immune response, from cellular development to organ and tissue formation exerting its effects through multiple interactions with both the innate and acquired branches of the immune system. It has been described at some extent changes in environment and nutrition produce dysbiosis in the gut but also in the skin, and lung microbiome, inducing qualitative and quantitative changes in composition and metabolic activity. Here we review the potential role of the skin, respiratory and gastrointestinal tract microbiomes in allergic diseases.In the gastrointestinal tract, the microbiome has been proven to be important in developing either effector or tolerant responses to different antigens by balancing the activities of Th1 and Th2 cells. In the lung, the microbiome may play a role in driving asthma endotype polarization, by adjusting the balance between Th2 and Th17 patterns. Bacterial dysbiosis is associated with chronic inflammatory disorders of the skin, such as atopic dermatitis and psoriasis. Thus, the microbiome can be considered a therapeutical target for treating inflammatory diseases, such as allergy. Despite some limitations, intervention

|
Suggested by
Société Francaise d'Immunologie
June 8, 2019 1:58 PM
|
Services on Demand Journal Article Indicators Cited by SciELO Access statistics Related links Cited by Google Similars in SciELO Similars in Google Share Anais Brasileiros de Dermatologia Print version ISSN 0365-0596On-line version ISSN 1806-4841 An. Bras. Dermatol. vol.94 no.2 supl.1 Rio de Janeiro Mar./Apr. 2019 Epub June 03, 2019 http://dx.doi.org/10.1590/abd1806-4841.2019940210 ; ATOPIC DERMATITIS Consensus on the therapeutic management of atopic dermatitis - Brazilian Society of Dermatology* 1Department of Dermatology, Faculdade de Medicina, Universidade de São Paulo, São Paulo (SP), Brazil. 2Dermatology Service, Irmandade Santa Casa de Misericórdia de Porto Alegre, Porto Alegre (RS), Brazil. 3Dermatology Service, Universidade Federal de Ciências da Saúde de Porto Alegre, Porto Alegre (RS), Brazil. 4Dermatology Service, Hospital de Clínicas de Porto Alegre, Universidade Federal do Rio Grande do Sul, Porto Alegre (RS), Brazil. 5Dermatology Service, Hospital das Clínicas, Universidade Federal de Minas Gerais, Belo Horizonte (MG), Brazil. 6Medical Dermatology Residency Program, Instituto de Ensino e Pesquisa, Hospital Sírio-Libanês, São Paulo (SP), Brazil. 7Clinic of Dermatology, Department of Medicine, Faculdade de Medicina da Santa Casa de São Paulo, São Paulo (SP), Brazil. 8Dermatology Service, Universidade do Estado do Pará, Belém (PA), Brazil. 9Dermatology Service, Hospital Municipal Jesus, Rio de Janeiro (RJ), Brazil. 10Dermatology Outpatient Clinic, Discipline of Dermatology, Faculdade de Medicina do ABC, Santo André (SP), Brazil. 11Dermatology Service, Hospital do Servidor Público Estadual, São Paulo (SP), Brazil. 12Dermatology Service, Complexo Hospitalar Padre Bento, Guarulhos (SP), Brazil. ABSTRACT: BACKGROUND: Atopic dermatitis is a highly prevalent inflammatory and pruritic dermatosis with a multifactorial etiology, which includes skin barrier defects, immune dysfunction, and microbiome alterations. Atopic dermatitis is mediated by genetic, environmental, and psychological factors and requires therapeutic management that covers all the aspects of its complex pathogenesis. OBJECTIVES: The aim of this article is to present the experience, opinions, and recommendations of Brazilian dermatology experts regarding the therapeutic management of atopic dermatitis. METHODS: Eighteen experts from 10 university hospitals with experience in atopic dermatitis were appointed by the Brazilian Society of Dermatology to organize a consensus on the therapeutic management of atopic dermatitis. The 18 experts answered an online questionnaire with 14 questions related to the treatment of atopic dermatitis. Afterwards, they analyzed the recent international guidelines on atopic dermatitis of the American Academy of Dermatology, published in 2014, and of the European Academy of Dermatology and Venereology, published in 2018. Consensus was defined as approval by at least 70% of the panel. RESULTS/CONCLUSION: The experts stated that the therapeutic management of atopic dermatitis is based on skin hydration, topical anti-inflammatory agents, avoidance of triggering factors, and educational programs. Systemic therapy, based on immunosuppressive agents, is only indicated for severe refractory disease and after failure of topical therapy. Early detection and treatment of secondary bacterial and viral infections is mandatory, and hospitalization may be needed to control atopic dermatitis flares. Novel target-oriented drugs such as immunobiologicals are invaluable therapeutic agents for atopic dermatitis. Keywords: Atopic dermatitis; Interleukins; Inflammation; Keratinocytes; Skin barrier INTRODUCTION Atopic dermatitis (AD) is a chronic inflammatory skin disease, with lesions showing typical morphology and distribution, and whose hallmark is intense pruritus. AD presents in patients with a personal or family history of atopic diseases such as asthma, rhinitis, or AD itself. It is one of the most frequent diseases of childhood, and its prevalence reaches up to 20% in infants and 2.1 to 4.9% in adults in Europe, North America, and Japan.1-3 Annual incidence of new cases of AD in patients below the age of 17 in the US is 11%; 85% of AD patients first manifest the disease before the age of 5, but 20-40% of children with AD persist with the skin disease in adulthood. 4,5 In the Brazilian population, prevalence of AD symptoms according to Solé et al. was 8.2% in children and 5.0% in adolescents. 6 Due to the complex pathogenesis of AD, which involves skin barrier defects, immune dysfunction, and microbiome alterations mediated by genetic, environmental, and psychological triggers, a single therapeutic approach is hardly capable of achieving disease control. 7 Increased transepidermal water loss (TEWL), decreased stratum corneum water content, and reduced expression of skin barrier proteins such as filaggrin and claudin 1 are the main alterations of the skin barrier in individuals with AD. 8-10 Of note is the cytokine dysregulation, leading to Th2, Th1, Th17, and Th22 polarization, which varies according to age, ethnicity, and AD phase. 11-13 Skin microbiome plays a crucial role in AD; about 90% of the skin of atopic individuals is colonized by Staphylococcus aureus (S. aureus). 14 The diversity of skin microbiome of AD patients shows temporal shifts, with a predominance of S. aureus during flares and Streptococcus, Propionibacterium, and Corynebacterium after treatment. 15 AD remains a challenging disease. Ideal treatment is targeted to long-term disease control with reduction of flares and maintenance of good quality of life. Moreover, treatment approaches depend on geographic, economic, and genotypic/phenotypic variations. This paper aims to communicate the experience, opinions, and recommendations of Brazilian dermatology experts on atopic dermatitis treatment. METHODS Eighteen faculty members from 10 university hospitals with expertise in AD were appointed by the Brazilian Society of Dermatology. The first step was the application of an online questionnaire with 14 questions regarding the management of AD patients by the experts at university hospitals. Table 1 shows the compiled answers. Table 1 answers/total Number of AD patients seen per month <50 9/18 >50 9/18 Number of patients seen in public institutions < 50 2 / 18 >50 16 / 18 Number of patients seen in private practice < 50 4 / 18 >50 13 / 18 Treatment based on published guidelines (12/18) American 5 / 12 European 5 / 12 Other 2 / 12 Topical therapy: Topical corticosteroids 17 / 18 Calcineurin inhibitors 16 / 18 Topical antibiotics 6 / 18 Other-moisturizers 7 / 18 Systemic therapy: Cyclosporine 14 / 18 Azathioprine 3 / 18 Methotrexate 11 / 18 Mycophenolatemofetil 1 / 18 Oral steroids 5 / 18 Immunobiologicaltherapy 0 / 18 Phototherapy (narrow-band UVB) Yes 15 / 18 No 3 / 18 Use of antimicrobials during flares Topical 10 / 18 Systemic 16 / 18 The second step was the analysis of recent international guidelines (American Academy of Dermatology, published in 2014, and the European Academy of Dermatology and Venereology, published in 2018). 16-21 All sections and recommendations regarding AD treatment were discussed with the 18 experts, and consensus was defined as approval by at least 70% of the panel. This paper expresses their opinions regarding international guidelines for AD treatment and provides practical guidance for dermatologists in Brazil. RESULTS/DISCUSSION Table 1 shows the data obtained from the applied questionnaire. The majority of experts (17/18) who answered the questionnaire work in public and private institutions. About 50% of the specialists see more than 50 AD patients/month, mostly at public hospitals. Twelve out of 18 of the dermatologists follow published consensuses, with emphasis on the American and European guidelines. The most widely used topical treatments are corticosteroids, followed by calcineurin inhibitors. The first choice for systemic therapy was cyclosporin, followed by methotrexate and azathioprine. Baseline therapy and preventive measures The recent guidelines are in accordance regarding baseline therapy. Key steps include maintenance of the skin barrier through the constant use of emollients, which recover the function of the damaged skin barrier in AD and consequently protect the skin from allergen penetration and subsequent inflammation. 22 Skin hydration improves xerosis and reduces pruritus, sparing topical corticosteroid use. Cleansing eliminates crusts and reduces bacterial contamination. The use of substances with physiological pH is recommended, and baths should last no longer than five minutes. 23 Sodium hypochlorite baths (bleach) may not always change the severity of AD but appear to reduce the need for topical anti-inflammatory drugs and antibiotics. 24 Daily bathing is possible for regular skin hydration, and emollients should be applied on slightly wet skin, immediately after drying; application twice daily is usually sufficient. 25 Some emollients have additional ingredients such as urea and propylene glycol, which may lead to skin irritation, and there is still inconclusive evidence about superiority of emollients enhanced with components of the skin barrier such as ceramides. 25,26 Recent concepts regarding the microbiome and the skin highlight that the cutaneous microbiome in AD is not as heterogeneous as in healthy individuals, with the predominance of Staphylococcus aureus (S. aureus). 15,27 Recovery of the skin barrier by adjusting the inflammatory response reestablishes the skin microbiome in AD patients. 28,29 Bacterial lysates or topical application of commensal bacteria are promising, but skin hydration itself is able to recover the skin microbiome. 15,30 There is evidence for early use of emollients in atopic dermatitis-prone children (three months of age and older) in the prevention of AD. 28,29 Recommendations by the dermatology experts for baseline therapy: Daily cleansing for up to 5 minutes with mild agents with adequate pH Emollient application twice daily on slightly wet skin is the main component of baseline therapy Aeroallergens Aeroallergens are relevant triggering factors of AD flares.31-33 Exacerbation of an eczematous lesion after skin contact or inhalation has been reported, but studies are still inconclusive. 32 The skin prick test and specific IgE are routinely utilized but have a low positive predictive value. 32 In the present panel of dermatology experts, 89% do not perform the skin prick test or RAST as part of routine practice. Food allergy One-third of the children with moderate/severe AD have associated food allergy; however, food allergy is not the cause of AD. 34 Restrictive diets should only be prescribed for children with proven food allergy. 34,35 The published guidelines recommend restrictive diets only for those patients with a positive oral challenge test, the gold standard assay for food allergy. 19,34-36 The detection of specific IgE to food through prick or serological tests does not prove food allergy, and their positive predictive value is low. 37 The present panel of dermatology experts does not recommend restrictive diets, but considers that food allergy may be investigated in children with severe, treatment-resistant AD and in those with a history of flares following ingestion of specific foods. Contact dermatitis Contact dermatitis is present in 40-65% of AD patients, usually exacerbating the existing eczema. 38 The patch test is recommended for refractory AD with atypical skin lesions. 39 Patients should be tested for fragrances, preservatives, topical corticosteroids, and other topical components. 38 Patients are more prone to develop occupational dermatoses, since AD exacerbates the irritant effect of allergens in certain professions such as hairdressers, mechanics, metalworkers, janitorial workers, and nurses, in whom hand eczema is commonly reported. 40 Preventive measures should be taken in order to reduce the incidence of AD in such patients. Fifty percent of the expert group recommend patch tests. The main problem is the difficulty in performing the test, since ideal sites are usually limited in AD patients. Topical anti-inflammatory therapy Topical anti-inflammatory therapy is the mainstay of AD treatment. Anti-inflammatory agents must have sufficient potency and should be applied on the skin lesions according to the recommendations and not exceeding the allowed amount per day. 41 Topical corticosteroids (TC) TC are the first line treatment for AD, with strong evidence of their superiority over placebo. 42 They are classified according to their potency based on vasoconstrictive effects, and every clinician should be aware of their potential local and systemic adverse effects, such as cutaneous atrophy and adrenal suppression.43,44 Strategies defining the use of TC vary according to their potency, but the suggested applied amounts of topical corticosteroids follow the fingertip unit rule.19,20 In the European guidelines, the approximate total amount of TC per month is 15g in infants, 30g in children, and 60-90g in adolescents and adults.20 The choice of corticosteroid and its vehicle depend on the affected site, the patients’ age and the severity and clinical phase of AD. Wet-wrap dressings may improve AD flares, and ultrahigh potent topical corticosteroids should be applied for up to two weeks.29,45 TC use depends on the vehicle; as a cream, they should be applied 15 minutes before the moisturizer, and as an ointment, applied 15 minutes prior to the moisturizer.41 Corticosteroid phobia is a relevant matter that should be addressed, especially due to its influence on adherence to treatment; it varies according to the country and culture.46 Topical corticosteroids are the first-line topical treatment for AD, according to the experts. Calcineurin inhibitors (topical immunomodulators or TIM) Tacrolimus and pimecrolimus are second-line non-corticosteroid, anti-inflammatory therapies for AD with proven efficacy.20,47 The most widely reported adverse effect is burning sensation during the initial days of use (especially with tacrolimus); they do not induce skin atrophy, which makes them useful for application on eyelids, perioral lesions, axillae, and genitals.48 Despite a black box warning in the package insert, studies have not reported an increased risk of lymphoma with the topical use of TIM at therapeutic doses.49 Intermittent use of TIM is recommended above two years of age.20 Eighty percent of the Brazilian experts use TIM as a second-line therapy for AD. Proactive treatment Proactive treatment has been proposed in published guidelines. It consists of long-term use of topical anti-inflammatory agents, either TC or TIM (tacrolimus), twice a week in previously affected areas, combined with moisturizers. 29,45,50,51 The rationale for proactive treatment is based on its efficacy and long-term safety (up to one year), reducing the number of flares and improving the quality of life of atopic patients. 50,51 The Brazilian experts recommend proactive treatment with TC or TIM in AD patients. Topical antimicrobial therapy Colonization by S. aureus is frequent on the skin of AD patients and is much higher than in non-atopic individuals (100% vs. 30%). 52-54Fortunately, the skin and nares of AD patients are not frequently colonized by methicillin-resistant S. aureus (MRSA) (7.4 and 4%, respectively). 54 The American Academy of Dermatology does not recommend the use of topical antibiotics, since they do not show clear benefits for AD patients. However, the use of 0.005% sodium chlorine in bathwater may be helpful in children and is recommended by the EADV. 17,20 During flares, 100% of the Brazilian experts use antibiotics. About 1/3 of the experts use topical antibiotics in acute phases of AD for short periods (up to one week). Recommendations for topical therapy in AD: TC are the first-line treatment for AD patients and must be carefully prescribed according to their potency and vehicle. Patient’s age, site, and phase of AD lesions are key factors when choosing TC. TIM constitute the second-line treatment for AD and are suitable for application on areas with high risk of corticosteroid-induced atrophy. Proactive therapy with either TC or TIM is safe, reduces flares and AD severity, and is indicated as long-term maintenance therapy. The use of topical antibiotics and antiseptics is still variable. Topical antibiotics can be used for short periods, and bleachers (0.005% sodium hypochlorite may be useful for pediatric AD). Wet-wrap bandages or occlusive treatment during hospitalization are helpful measures for improving flares. In patients that fail to respond to topical treatment, the following should be considered: -differential diagnoses of AD -lack of adherence -contact dermatitis -secondary infection (bacterial, viral, or fungal) -indication for systemic therapy Systemic treatment Systemic treatment of AD is recommended in moderate to severe cases that fail to respond topical therapies. Before initiating systemic treatment, it is mandatory to avoid aggravating factors, to diagnose and treat secondary infections, and to rule out differential diagnoses. The option for systemic therapy should also include the impact of the disease on the patient’s quality of life and a careful balance of risks and benefits with the chosen medication. 55,56 Phototherapy Phototherapy is a valid adjuvant therapeutic option, especially for chronic AD and in adults. It improves pruritus, thus reducing insomnia. 57 Ultraviolet B (UVB), narrow-band UVB, and psoralen + UVA (PUVA) are the main modalities. 12,57 UVB-NB (311-313nm) is the most widely used form and can be indicated for children. UVA1 (340-400nm) is seldom used in Brazil but is useful for flares. 21,57 In the Brazilian consensus group, 83% recommend this treatment modality, especially for the chronic phase of AD. Phototherapy improves clinical signs and reduces pruritus and bacterial colonization, thus being a steroid-sparing measure. It is important to avoid this treatment in patients with recurrent herpes simplex infection or history of eczema herpeticum. A limiting factor for this therapeutic modality is lack of adherence to long-term treatment. Antihistamines Oral antihistamines that block the histamine 1 receptor (H1R) have been prescribed for AD patients for decades; however, there are few randomized studies that evaluate their real efficacy in AD. 21 The aim of systemic antihistamines in AD is to allow better quality of sleep, since their role as anti-inflammatory agents in AD is controversial. There is no evidence of improvement of severity scores in randomized studies, and first-generation drugs are prescribed due to their sedative effect and to the relief of other conditions related to AD, such as asthma, rhinoconjunctivitis, dermographism, and urticaria. 21,58 However, our group stresses that the quality of sleep induced by anti-H1R drugs is not ideal, since they do not alter the REM phase. 21,58 Our group recommends the use of first-generation antihistamines (hydroxyzine and chlorpheniramine) based only on their sedative effect. Anti-inflammatory agents Cyclosporin A (CyA) Cyclosporin A is approved in many European countries and in Brazil for severe AD. The U.S. FDA approves it for psoriasis. The initial dose for children and adults varies from 3 to 5 mg/kg/day, and the maintenance dose is from 2.5 to 3 mg/kg/day. 55,59-61 Clinical improvement can be observed after 2-8 weeks; CyA is recommended for up to 2 years with constant monitoring of blood pressure and kidney function. 55,59-61 Periodic intervals of 3-6 months off therapy decreases the occurrence of side effects. 62 The average length of treatment with CyA is 3-12 months, and the drug is usually considered first-line treatment for treatment-resistant AD and in acute flares. 63 Pregnancy is not a contraindication to CyA use. 63 Although CyA leads to prompt improvement in severity scores after 2 weeks from the initial dose, reactivation of AD after the drug’s suspension is equally rapid, occurring in 2 weeks. 63 Methotrexate (MTX) MTX can be indicated as initial treatment for moderate/severe AD, recalcitrant to topical treatment with corticosteroids. The drug has a good safety profile and is indicated for long-term maintenance; clinical efficacy is reached after 8-12 weeks of administration.21,61,63 The therapeutic dose varies from 15 to 25mg/week for adults and 10-15mg/m2/week for children (oral, intravenous, or subcutaneous), and folate should be added to the treatment, usually 1-2 days after MTX. 21,61,63 Average length of treatment ranges from 6 to 12 months, and clinical improvement is seen at 8-12 weeks from the initial dose. Side effects include hematological disorders, liver enzyme alterations, and gastrointestinal discomfort. Its use is recommended for up to 2 years, with constant monitoring of bone marrow and liver function. 21,61,63,64 Contraception is mandatory, since the drug is considered category X. 61,63 Azathioprine (AZA) AZA can be indicated as systemic treatment for refractory AD. Peak efficacy of AZA is reached after 8-12 weeks of use. 63 The initial dose is usually 50 mg/day for 1-2 weeks, increased thereafter to 2-3 mg/kg/day. 63,65 It can increase the risk of non-melanoma skin cancer and lymphoma. 66,67 Thiopurine methyltransferase enzyme (TPMT) levels should be measured whenever possible, since TPMT deficiency while in use of AZA can lead to bone marrow aplasia. 65 It can be prescribed for children (off label for AD) and is subject to restricted indication during pregnancy. 63,68 Mycophenolate mofetil (MMF) Clinical efficacy of MMF is reached after 8-12 weeks of use (off label in AD), and the drug has a good safety profile. 21,63 The recommended doses in adults are 1-2g/day (starting dose) and 2-3g/ day (maintenance); the pediatric doses are 20-50mg/kg/day (starting dose) and 30-50mg/kg/day (maintenance).21,68 Gastrointestinal and hematological side effects have been reported. 21,63 Systemic corticosteroids (SC) There are few randomized controlled studies regarding the use of systemic corticosteroids in AD. In the 2018 European consensus, SC are used in exceptional cases of AD, but only for one week.21 There is a rapid clear up of skin lesions , but severe rebound tends to occur in 2 weeks. 21 One controlled trial indicates lower efficacy of systemic prednisolone in comparison to CyA in severe AD.21,69 Position/recommendations for the use of systemic anti-inflammatory drugs in AD: CyA and MTX are the most widely used systemic drugs for severe refractory AD. CyA leads to fast improvement of AD severity scores after 2 weeks of initial treatment, but reactivation of AD after drug suspension is equally fast, occurring in 2 weeks. MTX can be used as the initial systemic medication for refractory moderate/severe AD and is indicated for long-term maintenance. Clinical efficacy is reached after 8-12 weeks of administration. Oral corticosteroids are used in exceptional cases for short periods (up to 1 week). Few dermatologists have experience with mycophenolate mofetil or azathioprine. Treatment of secondary infections (bacterial, viral, or fungal) S. aureus and Streptococcus pyogenes are the most common bacterial agents in AD. They are detected in more than 90% of AD lesions.53 Systemic antibiotics are reserved for patients with clinical evidence of infection, and cephalosporins are the first choice of treatment.21,70 Extensive viral infections such as eczema herpeticum (EH) are seen in AD patients. Skin barrier defects, including mutations of the filaggrin and claudin1 genes or abnormalities in IFN-gamma response may increase the risk of EH. 12,71 Risk factors for EH include early severe AD, high IgE levels, eosinophilia, and associated food allergy and asthma.14,45,63,72 Treatment for localized EH is oral acyclovir. Systemic involvement with fever, lethargy, headache, nausea, and dizziness requires hospitalization and intravenous acyclovir. 72 As for fungal infections in patients with AD, Malassezia spp. appears to contribute to skin inflammation during flares, and there is an anti-IgE response to immunogenic proteins released by some Malassezia species. 73 Recommendations by the Brazilian experts: oral antibiotics are indicated when there are signs of bacterial superinfection of the skin; cephalosporins are the first choice, followed by sulfamethoxazole-trimethoprim. Eczema herpeticum must be treated with systemic antiviral drugs; when it is followed by systemic symptoms and signs, hospitalization and intravenous antiviral therapy are indicated. AD patients with head and neck involvement may benefit from treatment with antifungal agents. Education and AD AD has a strong impact on the quality of life of patients and caregivers due to its chronic course and intense pruritus. 21,45,74 Sleep loss, school and work absenteeism, social isolation, depression, and suicidal ideation may be present. 56,74 Low treatment adherence is common in AD, and educational programs are needed to reinforce the patient’s understanding of the disease complexity and therapeutic approaches. 75 Various models focusing on AD education and with multidisciplinary approaches have shown subjective and objective improvement of AD worldwide. 75-79 Future perspectives Immunobiologicals and small molecules are targeted therapies that have been developed for many inflammatory, autoimmune, and oncologic diseases. Crisaborole ointment is a topical phosphodiesterase 4 (PDE4) inhibitor that was approved in the USA in 2017 for patients above the age of 2 years with mild/moderate AD. 80 Dupilumab is a human monoclonal antibody for AD that blocks the alfa-chain receptor for IL-4 and IL-13 (dupilumab) and is approved for adults with moderate/severe AD. 81 Its efficacy after 16 weeks as monotherapy (initial dose: 600 mg, followed by 300 mg every 2 weeks, SC), measured by the reduction of eczema severity scores (EASI) was 82.5% (EASI 50), 60.3% (EASI 75), and 36.5% (EASI 90).81-83 Improvement of skin lesions and reduction of pruritus improved 2 weeks after initiating treatment. 81-83The studies show sustained long-term efficacy (one year) with dupilumab combined with TC in AD patients.84,85 The main adverse event reported with dupilumab was conjunctivitis, detected in 25-50% of AD patients.85,86 There are ongoing studies (phases 2-3) with novel immunobiologicals and small molecules for AD treatment. See Chart 1. 87,88 Chart 1 Agent Target Administration Phase Tralokinumab IL-13 SC 3 Lebrikizumab IL-13 SC 2 Nemolizumab IL-31Ra SC 3 Apremilast PDE4 PO 2 ILV-094 IL-22 IV terminated Secukinumab IL-17 SC 2 Baricitinib JAK1/2 PO 2 Upadacitinib JAK1 SC 2 ZPL389 H4R PO 2 Tezepelumab TSLP SC 2 Serlopitant NKR1 PO 2 IL=interleukin; R=receptor PDE=phosphodiesterase; JAK= janus kinase; H=histamine; TSLP=thymic stromal lymphopoietin; NKR=neurokinin receptor; SC= subcutaneously; PO= per oral; IV= intravenously Source: Wang, et al, 201687 and Lee, et al, 2018.88 Chart 1: New systemic drugs for AD treatment. 87,88 CONCLUSIONS Despite the cultural and economic differences between Brazil, USA, and Europe, including in access to immunobiological therapies, the ideal management of AD is based on a better understanding of disease pathogenesis and knowledge of treatment strategies. Basic treatment for AD includes skin hydration, topical anti-inflammatory therapy, avoidance of aggravating factors, and educational programs with a multidisciplinary approach. Systemic therapy should be only indicated for refractory or severe disease after attempts with topical therapy. Secondary infections must be diagnosed early and treated promptly, and hospitalization may be necessary to control flares (Figure 1). Figure 1 Novel target-oriented drugs are invaluable tools for AD treatment. *Work conducted at the Sociedade Brasileira de Dermatologia, Rio de Janeiro (RJ), Brazil. Financial support: None. REFERENCES 1 Eichenfield LF, Ellis CN, Mancini AJ, Paller AS, Simpson EL. Atopic dermatitis: epidemiology and pathogenesis update. Semin Cutan Med Surg. 2012;31(3 Suppl):S3-5. [ Links ] 2 Watson W, Kapur S. Atopic dermatitis. Allergy Asthma Clin Immunol. 2011;7( Suppl 1):S4 [ Links ] 3 Barbarot S, Auziere S, Gadkari A, Girolomoni G, Puig L, Simpson EL, et al. Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy. 2018;73:1284-1293 [ Links ] 4 Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112(6 Suppl):S118-27. [ Links ] 5 Ozkaya E. Adult-onset atopic dermatitis. J Am Acad Dermatol. 2005;52:579-82. [ Links ] 6 S Solé D, Camelo-Nunes IC, Wandalsen GF, Mallozi MC, Naspitz CK; Brazilian ISAAC Group. Prevalence of atopic eczema and related symptoms in Brazilian schoolchildren: results from the International Study of Asthma and Allergies in Childhood (ISAAC) phase 3. J Investig Allergol Clin Immunol. 2006;16:367-76. [ Links ] 7 Orfali RL, Zaniboni MC, Aoki V. Profile of skin barrier proteins and cytokines in adults with atopic dermatitis. G Ital Dermatol Venereol. 2017;152:140-7. [ Links ] 8 Batista DI, Perez L, Orfali RL, Zaniboni MC, Samorano LP, Pereira NV, et al. Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J Eur Acad Dermatol Venereol. 2015;29:1091-5. [ Links ] 9 Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008;121:1337-43. [ Links ] 10 Gittler JK, Shemer A, Suárez-Fariñas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130:1344-54. [ Links ] 11 Czarnowicki T, Gonzalez J, Shemer A, Malajian D, Xu H, Zheng X et al. Severe atopic dermatitis is characterized by selective expansion of circulating TH2/ TC2 and TH22/TC22, but not TH17/TC17, cells within the skin-homing T-cell population. J Allergy Clin Immunol. 2015;136:104-115.e7. [ Links ] 12 Leung DY. New insights into atopic dermatitis: role of skin barrier and immune dysregulation. Allergol Int. 2013;62:151-61. [ Links ] 13 Shi B, Bangayan NJ, Curd E, Taylor PA, Gallo RL, Leung DYM, et al. The skin microbiome is different in pediatric versus adult atopic dermatitis. J Allergy Clin Immunol. 2016;138:1233-6. [ Links ] 14 Ong PY, Leung DY. Bacterial and Viral Infections in Atopic Dermatitis: a Comprehensive Review. Clin Rev Allergy Immunol. 2016;51:329-37. [ Links ] 15 Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850-9. [ Links ] 16 Eichenfield LF, Tom WL, Chamlin SL, Feldman SR, Hanifin JM, Simpson EL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-51. [ Links ] 17 Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-32. [ Links ] 18 Sidbury R, Davis DM, Cohen DE, Cordoro KM, Berger TG, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-49 [ Links ] 19 Sidbury R, Tom WL, Bergman JN, Cooper KD, Silverman RA, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: Section 4. Prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014;71:1218-33. [ Links ] 20 Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657-82. [ Links ] 21 Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32:850-78. [ Links ] 22 Åkerström U, Reitamo S, Langeland T, Berg M, Rustad L, Korhonen L, et al. Comparison of Moisturizing Creams for the Prevention of Atopic Dermatitis Relapse: A Randomized Double-blind Controlled Multicentre Clinical Trial. Acta Derm Venereol. 2015;95:587-92. [ Links ] 23 Blume-Peytavi U, Cork MJ, Faergemann J, Szczapa J, Vanaclocha F, Gelmetti C. Bathing and cleansing in newborns from day 1 to first year of life: recommendations from a European round table meeting. J Eur Acad Dermatol Venereol. 2009;23:751-9. [ Links ] 24 Hon KL, Tsang YC, Lee VW, Pong NH, Ha G, Lee ST, et al. Efficacy of sodium hypochlorite (bleach) baths to reduce Staphylococcus aureus colonization in childhood onset moderate-to-severe eczema: A randomized, placebo-controlled cross-over trial. J Dermatolog Treat. 2016;27:156-62. [ Links ] 25 Koutroulis I, Pyle T, Kopylov D, Little A, Gaughan J, Kratimenos P. The Association Between Bathing Habits and Severity of Atopic Dermatitis in Children. Clin Pediatr (Phila). 2016;55:176-81. [ Links ] 26 Blume-Peytavi U, Lavender T, Jenerowicz D, Ryumina I, Stalder JF, Torrelo A, et al. Recommendations from a European Roundtable Meeting on Best Practice Healthy Infant Skin Care. Pediatr Dermatol. 2016;33:311-21. [ Links ] 27 Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190-2. [ Links ] 28 Simpson EL, Chalmers JR, Hanifin JM, Thomas KS, Cork MJ, McLean WH, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-23. [ Links ] 29 Mohan GC, Lio PA. Comparison of Dermatology and Allergy Guidelines for Atopic Dermatitis Management. JAMA Dermatol. 2015;151:1009-13. [ Links ] 30 Myles IA, Earland NJ, Anderson ED, Moore IN, Kieh MD, Williams KW, et al. First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight. 2018;3. pii: 120608 [ Links ] 31 Werfel T, Heratizadeh A, Niebuhr M, Kapp A, Roesner LM, Karch A, et al. Exacerbation of atopic dermatitis on grass pollen exposure in an environmental challenge chamber. J Allergy Clin Immunol. 2015;136:96-103.e9. [ Links ] 32 Garritsen FM, ter Haar NM, Spuls PI. House dust mite reduction in the management of atopic dermatitis. A critically appraised topic. Br J Dermatol. 2013;168:688-91 [ Links ] 33 Lorenzini D, Pires M, Aoki V, Takaoka R, Souza RL, Vasconcellos C. Atopy patch test with Aleuroglyphus ovatus antigen in patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2015;29:38-41. [ Links ] 34 Eigenmann PA, Sicherer SH, Borkowski TA, Cohen BA, Sampson HA. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998;101:E8. [ Links ] 35 Bath-Hextall F, Delamere FM, Williams HC. Dietary exclusions for improving established atopic eczema in adults and children: systematic review. Allergy. 2009;64:258-64. [ Links ] 36 Thompson MM, Tofte SJ, Simpson EL, Hanifin JM. Patterns of care and referral in children with atopic dermatitis and concern for food allergy. Dermatol Ther. 2006;19:91-6. [ Links ] 37 Cuomo B, Indirli GC, Bianchi A, Arasi S, Caimmi D, Dondi A, et al. Specific IgE and skin prick tests to diagnose allergy to fresh and baked cow's milk according to age: a systematic review. Ital J Pediatr. 2017;43:93. [ Links ] 38 Herro EM, Matiz C, Sullivan K, Hamann C, Jacob SE. Frequency of contact allergens in pediatric patients with atopic dermatitis. J Clin Aesthet Dermatol. 2011;4:39-41. [ Links ] 39 Arkwright PD, Motala C, Subramanian H, Spergel J, Schneider LC, Wollenberg A, et al. Management of difficult-to-treat atopic dermatitis. J Allergy Clin Immunol Pract. 2013;1:142-51. [ Links ] 40 Borok J, Matiz C, Goldenberg A, Jacob SE. Contact Dermatitis in Atopic Dermatitis Children-Past, Present, and Future. Clin Rev Allergy Immunol. 2018. [Epub ahead of print] [ Links ] 41 Ring J, Alomar A, Bieber T, Deleuran M, Fink-Wagner A, Gelmetti C, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) part I. J Eur Acad Dermatol Venereol. 2012;26:1045-60 [ Links ] 42 Van Der Meer JB, Glazenburg EJ, Mulder PG, Eggink HF, Coenraads PJ. The management of moderate to severe atopic dermatitis in adults with topical fluticasone propionate. The Netherlands Adult Atopic Dermatitis Study Group. Br J Dermatol. 1999;140:1114-21. [ Links ] 43 Luger TA. Balancing efficacy and safety in the management of atopic dermatitis: the role of methylprednisolone aceponate. J Eur Acad Dermatol Venereol. 2011;25:251-8. [ Links ] 44 Walsh P, Aeling JL, Huff L, Weston WL. Hypothalamus-pituitary-adrenal axis suppression by superpotent topical steroids. J Am Acad Dermatol. 1993;29:501-3. [ Links ] 45 Lebwohl MG, Del Rosso JQ, Abramovits W, Berman B, Cohen DE, Guttman E, et al. Pathways to managing atopic dermatitis: consensus from the experts. J Clin Aesthet Dermatol. 2013;6(7 Suppl):S2-S18. [ Links ] 46 Stalder JF, Aubert H, Anthoine E, Futamura M, Marcoux D, Morren MA et al. Topical corticosteroid phobia in atopic dermatitis: International feasibility study of the TOPICOP score. Allergy. 2017;72:1713-9. [ Links ] 47 Alomar A, Berth-Jones J, Bos JD, Giannetti A, Reitamo S, Ruzicka T, et al. The role of topical calcineurin inhibitors in atopic dermatitis. Br J Dermatol. 2004;151(Suppl 70):S3-27. [ Links ] 48 Reitamo S, Rissanen J, Remitz A, Granlund H, Erkko P, Elg P, et al. Tacrolimus ointment does not affect collagen synthesis: results of a single-center randomized trial. J Invest Dermatol. 1998;111:396-8. [ Links ] 49 Arellano FM, Wentworth CE, Arana A, Fernández C, Paul CF.. Risk of lymphoma following exposure to calcineurin inhibitors and topical steroids in patients with atopic dermatitis. J Invest Dermatol. 2007;127:808-16. [ Links ] 50 Thaçi D, Reitamo S, Gonzalez Ensenat MA, Moss C, Boccaletti V, Cainelli T, et al. Proactive disease management with 0.03% tacrolimus ointment for children with atopic dermatitis: results of a randomized, multicentre, comparative study. Br J Dermatol. 2008;159:1348-56. [ Links ] 51 Wollenberg A, Reitamo S, Girolomoni G, Lahfa M, Ruzicka T, Healy E, et al. Proactive treatment of atopic dermatitis in adults with 0.1% tacrolimus ointment. Allergy. 2008;63:742-50. [ Links ] 52 Orfali RL, Rivitti E, Sato MN, Takaoka R, Aoki V. Adult atopic dermatitis: Evaluation of TH17 and TH22 cytokines in peripheral blood mononuclear cells induced by staphylococcal enterotoxins A and B. J Am Acad Dermatol. 2012;66:AB68-AB. [ Links ] 53 Breuer K, Wittmann M, Kempe K, Kapp A, Mai U, Dittrich-Breiholz O, et al. Alphatoxin is produced by skin colonizing Staphylococcus aureus and induces a T helper type 1 response in atopic dermatitis. Clin Exp Allergy. 2005;35:1088-95. [ Links ] 54 Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics. 2009;123:e808-14. [ Links ] 55 Simpson EL, Bruin-Weller M, Flohr C, Ardern-Jones MR, Barbarot S, Deleuran M, et al. When does atopic dermatitis warrant systemic therapy? Recommendations from an expert panel of the International Eczema Council. J Am Acad Dermatol. 2017;77:623-633. [ Links ] 56 Ring J, Alomar A, Bieber T, Deleuran M, Fink-Wagner A, Gelmetti C, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) Part II. J J Eur Acad Dermatol Venereol. 2012;26:1176-93 [ Links ] 57 Garritsen FM, Brouwer MW, Limpens J, Spuls PI. Photo(chemo)therapy in the management of atopic dermatitis: an updated systematic review with implications for practice and research. Br J Dermatol. 2014;170:501-13. [ Links ] 58 Diepgen TL; Early Treatment of the Atopic Child Study Group. Long-term treatment with cetirizine of infants with atopic dermatitis: a multi-country, double-blind, randomized, placebo-controlled trial (the ETAC trial) over 18 months. Pediatr Allergy Immunol. 2002;13:278-86. [ Links ] 59 Brasch J, Becker D, Aberer W, Bircher A, Kränke B, Jung K, et al. Guideline contact dermatitis: S1-Guidelines of the German Contact Allergy Group (DKG) of the German Dermatology Society (DDG), the Information Network of Dermatological Clinics (IVDK), the German Society for Allergology and Clinical Immunology (DGAKI), the Working Group for Occupational and Environmental Dermatology (ABD) of the DDG, the Medical Association of German Allergologists (AeDA), the Professional Association of German Dermatologists (BVDD) and the DDG. Allergo J Int. 2014;23:126-38. [ Links ] 60 van der Schaft J, Politiek K, van den Reek JM, Christoffers WA, Kievit W, de Jong EM, et al. Drug survival for ciclosporin A in a long-term daily practice cohort of adult patients with atopic dermatitis. Br J Dermatol. 2015;172:1621-7. [ Links ] 61 Goujon C, Viguier M, Staumont-Sallé D, Bernier C, Guillet G, Lahfa M, et al. Methotrexate Versus Cyclosporine in Adults with Moderate-to-Severe Atopic Dermatitis: A Phase III Randomized Noninferiority Trial. J Allergy Clin Immunol Pract. 2018;6:562-569.e3. [ Links ] 62 van der Schaft J, van Zuilen AD, Deinum J, Bruijnzeel-Koomen CA, de Bruin-Weller MS. Serum creatinine levels during and after long-term treatment with cyclosporine A in patients with severe atopic dermatitis. Acta Derm Venereol. 2015;95:963-7. [ Links ] 63 Wollenberg A, Oranje A, Deleuran M, Simon D, Szalai Z, Kunz B, et al. ETFAD/ EADV Eczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients. J Eur Acad Dermatol Venereol. 2016;30:729-47. [ Links ] 64 Schneider L, Tilles S, Lio P, Boguniewicz M, Beck L, LeBovidge J, et al. Atopic dermatitis: a practice parameter update 2012. J Allergy Clin Immunol. 2013;131:295-9.e1-27. [ Links ] 65 Meggitt SJ, Gray JC, Reynolds NJ. Azathioprine dosed by thiopurine methyltransferase activity for moderate-to-severe atopic eczema: a double-blind, randomised controlled trial. Lancet. 2006;367:839-46. [ Links ] 66 Khan N, Abbas AM, Lichtenstein GR, Loftus EV Jr, Bazzano LA. Risk of lymphoma in patients with ulcerative colitis treated with thiopurines: a nationwide retrospective cohort study. Gastroenterology. 2013;145:1007-1015.e3. [ Links ] 67 Peyrin-Biroulet L, Khosrotehrani K, Carrat F, Bouvier AM, Chevaux JB, Simon T, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141:1621-28.e1-5. [ Links ] 68 Waxweiler WT, Agans R, Morrell DS. Systemic treatment of pediatric atopic dermatitis with azathioprine and mycophenolate mofetil. Pediatr Dermatol. 2011;28:689-94 [ Links ] 69 Schmitt J, Schäkel K, Fölster-Holst R, Bauer A, Oertel R, Augustin M, et al. Prednisolone vs. ciclosporin for severe adult eczema. An investigator-initiated double-blind placebo-controlled multicentre trial. Br J Dermatol. 2010;162:661-8. [ Links ] 70 Park HY, Kim CR, Huh IS, Jung MY, Seo EY, Park JH, et al. Staphylococcus aureus Colonization in Acute and Chronic Skin Lesions of Patients with Atopic Dermatitis. Ann Dermatol. 2013;25:410-6. [ Links ] 71 Leung DY, Gao PS, Grigoryev DN, Rafaels NM, Streib JE, Howell MD, et al. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-gamma response. J Allergy Clin Immunol. 2011;127:965-73.e1-5. [ Links ] 72 Sun D, Ong PY. Infectious Complications in Atopic Dermatitis. Immunol Allergy Clin North Am. 2017;37:75-93. [ Links ] 73 Glatz M, Bosshard P, Schmid-Grendelmeier P. The Role of Fungi in Atopic Dermatitis. Immunol Allergy Clin North Am. 2017;37:63-74. [ Links ] 74 Gieler U, Köhnlein B, Schauer U, Freiling G, Stangier U. Counseling of parents with children with atopic dermatitis. Hautarzt. 1992;43(Suppl 11):S37-42. [ Links ] 75 Staab D, von Rueden U, Kehrt R, Erhart M, Wenninger K, Kamtsiuris P, et al. Evaluation of a parental training program for the management of childhood atopic dermatitis. Pediatr Allergy Immunol. 2002;13:84-90. [ Links ] 76 Mancini AJ, Paller AS, Simpson EL, Ellis CN, Eichenfield LF. Improving the patient-clinician and parent-clinician partnership in atopic dermatitis management. Semin Cutan Med Surg. 2012;31(3 Suppl):S23-8 [ Links ] 77 Kupfer J, Gieler U, Diepgen TL, Fartasch M, Lob-Corzilius T, Ring J, et al. Structured education program improves the coping with atopic dermatitis in children and their parents-a multicenter, randomized controlled trial. J Psychosom Res. 2010;68:353-8. [ Links ] 78 Takaoka R, Aoki V. Education of Patients with Atopic Dermatitis and Their Caregivers. Pediatric Allergy Immunology and Pulmonology. 2016;29:160-3. [ Links ] 79 Weber MB, Lorenzini D, Reinehr CP, Lovato B. Assessment of the quality of life of pediatric patients at a center of excellence in dermatology in southern Brazil. An Bras Dermatol. 2012;87:697-702. [ Links ] 80 Paller AS, Tom WL, Lebwohl MG, Blumenthal RL, Boguniewicz M, Call RS, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6. [ Links ] 81 Simpson EL, Gadkari A, Worm M, Soong W, Blauvelt A, Eckert L, et al. Dupilumab therapy provides clinically meaningful improvement in patient-reported outcomes (PROs): A phase IIb, randomized, placebo-controlled, clinical trial in adult patients with moderate to severe atopic dermatitis (AD). J J Am Acad Dermatol. 2016;75:506-15. [ Links ] 82 Thaçi D, Simpson EL, Beck LA, Bieber T, Blauvelt A, Papp K, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387:40-52. [ Links ] 83 Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N Engl J Med. 2016;375:2335-48. [ Links ] 84 Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139(4 Suppl):S65-76. [ Links ] 85 Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, Weisman J, Pariser D, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303. [ Links ] 86 Wollenberg A, Ariens L, Thurau S, van Luijk C, Seegräber M, de Bruin-Weller M. Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab-clinical characteristics and treatment. J Allergy Clin Immunol Pract. 2018;6:1778-80.e1 [ Links ] 87 Wang D, Beck LA. Immunologic Targets in Atopic Dermatitis and Emerging Therapies: An Update. Am J Clin Dermatol. 2016;17:425-43. [ Links ] 88 Lee DE, Clark AK, Tran KA, Shi VY. New and emerging targeted systemic therapies: a new era for atopic dermatitis. J Dermatolog Treat. 2018;29:364-74. [ Links ] Received: October 22, 2018; Accepted: January 13, 2019 Mailing address: Valeria Aoki. E-mail: valeria.aoki@gmail.com Daniel Lorenzini. E-mail: daniellorenzini@gmail.com Conflict of interest: None. AUTHORS’ CONTRIBUTIONS Valeria Aoki 0000-0003-4256-4413 Approval of the final version of the manuscript; Conception and planning of the study; Elaboration and writing of the manuscript; Obtaining, analyzing and interpreting the data; Critical review of the literature; Critical review of the manuscript Daniel Lorenzini 0000-0002-6850-5799 Approval of the final version of the manuscript; Elaboration and writing of the manuscript; Obtaining, analyzing and interpreting the data; Critical review of the literature; Critical review of the manuscript Raquel Leão Orfali 0000-0002-2807-1404 Approval of the final version of the manuscript; Obtaining, analyzing and interpreting the data; Critical review of the literature; Critical review of the manuscript Mariana Colombini Zaniboni 0000-0002-7830-8668 Approval of the final version of the manuscript; Obtaining, analyzing and interpreting the data; Critical review of the literature; Critical review of the manuscript Zilda Najjar Prado de Oliveira 0000-0002-8596-1999 Approval of the final version of the manuscript; Critical review of the manuscript Maria Cecília Rivitti-Machado 0000-0003-2910-7330 Approval of the final version of the manuscript; Critical review of the manuscript Roberto Takaoka 0000-0003-0952-2641 Approval of the final version of the manuscript; Critical review of the manuscript Magda Blessmann Weber 0000-0001-5885-5851 Approval of the final version of the manuscript; Critical review of the manuscript Tania Cestari 0000-0003-3001-0202 Approval of the final version of the manuscript; Critical review of the manuscript Bernardo Gontijo 0000-0003-1938-5986 Approval of the final version of the manuscript; Critical review of the manuscript Andrea Machado Coelho Ramos 0000-0001-7414-3395 Approval of the final version of the manuscript; Critical review of the manuscript Claudia Marcia de Resende Silva 0000-0002-3250-1227 Approval of the final version of the manuscript; Critical review of the manuscript Silmara da Costa Pereira Cestari 0000-0001-9824-7906 Approval of the final version of the manuscript; Critical review of the manuscript Silvia Souto-Mayor 0000-0001-9335-2758 Approval of the final version of the manuscript; Critical review of the manuscript Francisca Regina Carneiro 0000-0001-6735-4004 Approval of the final version of the manuscript; Critical review of the manuscript Ana Maria Mosca de Cerqueira 0000-0001-8779-834X Approval of the final version of the manuscript; Critical review of the manuscript Cristina Laczynski 0000-0001-7483-5826 Approval of the final version of the manuscript; Critical review of the manuscript Mario Cezar Pires 0000-0001-7587-8932 Approval of the final version of the manuscript; Elaboration and writing of the manuscript; Critical review of the manuscript ©2019 by Anais Brasileiros de Dermatologia This is an Open Access article distributed under the terms of the Creative Commons Attribution NonCommercial License which permits unrestricted noncommercial use, distribution, and reproduction in any medium provided the original work is properly cited.

|
Scooped by
Gilbert C FAURE
May 22, 2019 7:41 AM
|

|
Scooped by
Gilbert C FAURE
May 3, 2019 8:35 AM
|
Component-resolved diagnosis (CRD) allows to identify single molecular allergen components, and constitutes a routine practice in many allergy units. However, skin prick test (SPT) remains the technique of choice in many otorhinolaryngology departments, thus increasing the risk of using inadequate immunotherapies in patients with respiratory allergies. This study aimed to compare sensitization profiles determined by SPT and CRD in patients with respiratory allergy, and to explore the relationship between sensitization and type and severity of the respiratory disease. Cross-sectional, multicenter study of patients admitted to the Otorhinolaryngology Department due to symptoms of respiratory allergy. Extracts from various house dust mites, pollens, and molds were tested by SPT, whereas IgE against the corresponding antigens were measured by CRD. The analysis included 101 patients. The sensitization profile obtained by SPT had low agreement with that of CRD, particularly to dust mite allergens (Dermatophagoides sp.) and pollens (Plantago lanceolata, Olea europaea, and Cupressus sempervirens). While SPT did not show any significant relationship between sensitization and type/severity of the respiratory disease, CRD allowed to associate Der p 1, Der f 1 and Lep d 2 sensitizations with asthma, and Der p 2, Der f 2 and Lep d 2 sensitizations with more severe symptoms of allergic rhinitis. Compared with SPT, CRD enables to describe a more accurate sensitization profile and to identify associations between symptoms and specific antigens. The routine use of CRD in an otorhinolaryngology setting may benefit the management of patients with respiratory allergy. Trial registration IB 3108/15 (Retrospectively registered)

|
Scooped by
Gilbert C FAURE
April 29, 2019 6:54 AM
|
According to a current study of the Medical University Vienna, specialized T memory lymphocytes in the lungs that react to inhaled allergens, cause attacks of allergic asthma.

|
Scooped by
Gilbert C FAURE
April 18, 2019 7:50 AM
|
A large, multi-ethnic genome-wide association study (GWAS) of asthma identified novel associations with potential relevance for asthma susceptibility in older adults of diverse racial backgrounds.

|
Suggested by
Société Francaise d'Immunologie
March 19, 2019 3:41 PM
|
Home: Education and Programs: Education: Allergic Disease Resource Center: Professionals: The Role of Cytokines in the Inflammatory Process of Asthma and Response to Therapy The Role of Cytokines in the Inflammatory Process of Asthma and Response to Therapy Posted: 2008 Robert G. Townley, M.D. Professor, Medicine, Medical Microbiology and Immunology Creighton University 601 N. 30 Street, Suite 3M-100 Omaha, NE 68131 402-280-1839 (phone) 402-280-4115 (fax) e-mail: rtownley@creighton.edu The Contribution of Inflammatory Processes to the Characteristics of Asthma Introduction Asthma is a complex disease involving many different inflammatory cells, cytokines and chemokines that result in structural changes, remodeling and ultimately in the signs and symptoms of asthma. The purpose of this synopsis is to focus on the inflammatory processes contributing to the pathogenesis of asthma, and on the role of corticosteroids on inflammation, remodeling and their effect on profibrotic cytokines and vascular endothelial growth factor (VEGF) in severe asthma. The effect of antigen challenge on the production of proinflammatory cytokines and subsequent reduction in the bronchodilating effect of beta-adrenergic agents is reviewed. (1,2,3) The effect of cytokines, corticosteroids and thermoplasty on airway smooth muscle and the immunological and clinical changes following treatment with omalizumab will be discussed. The fact that IL-13 causes airway hyperresponsiveness and decreased responsiveness to beta-adrenergic agonists and corticosteroids emphasizes the importance of the development of IL-13 antagonists for asthma therapy. (1,2,4) Asthma is characterized by bronchoconstriction, bronchial hyperresponsiveness, a decreased response to ß-adrenergic agents and inflammation. The inflammation is marked by pulmonary airway eosinophilia, increased exhaled nitric oxide, and the expression of specific T-cell cytokines including interleukin-4 (IL-4), IL-5, IL-9 and IL-13. (5) Sputum from patients with asthma is characterized by tight spirals of mucus that originate from the small bronchioles and by small mucus plugs that are typical of bronchopulmonary aspergillosis. Bronchial obstruction and bronchoconstriction leads to dyspnea, wheezing and chest cough. In addition to chronic inflammation, asthma is characterized by structural alterations in the airways that together are called airway remodeling. Airway remodeling in the large and small airways includes subepithelial thickening, epithelial denudation with goblet cell metaplasia, increased airway smooth muscle mass, bronchial gland enlargement, angiogenesis and alterations in the extracellular matrix components (5). This remodeling is thought to be initiated by inflammation of the airways. (6) Complex interactions between airway inflammation, structural changes and airway hyperresponsiveness occur in mice sensitized by chronic exposure to allergens. Patients who exhibit persistent airway hyperresponsiveness with fixed airflow limitation may have significant airway narrowing despite maximal therapy; components of airway remodeling are likely to contribute to airway hyperresponsiveness, airflow limitation, and airway narrowing. (5,6) The Effect of Corticosteroids on Inflammation and Airway Remodeling Corticosteroids have been the most extensively evaluated asthma therapy. Epithelial denudation increases the exposure of mucosal nerve endings, enhances the penetration of allergens and reduces mucocilliary clearance. Biopsy studies have demonstrated that corticosteroids partially restore the epithelial lining of the mucosa. (7) Goblet cell metaplasia and mucus hypersecretion are also characteristic features of asthma, and corticosteroids are effective in reducing goblet cell metaplasia in animal models. Studies of basement membrane components and thickness have been difficult to interpret, and the consequences of basement membrane remodeling in asthma remain controversial. (5,6) Some investigators have shown that the thickness of the collagen is related to airway obstruction and airway responsiveness. Others have argued that these findings are of little consequence. However, fibrosis of airway smooth muscle is more likely to have functional consequences. (6) The lamina propria may be thickened in asthma, and inhaled corticosteroids are effective in preventing and reversing the enhanced fibronectin deposition that occurs during repeated exposure to allergen. However, treatment with inhaled steroids at low doses or after allergen exposure does not affect fibronectin deposition and suggests that the dose of inhaled corticosteroids may be critical. (5) Treatment of patients with asthma with inhaled corticosteroids for ten years markedly decreased inflammation. It is interesting however, that this reduced inflammation was not always associated with improvement of bronchial hyperresponsiveness. In a prospective study, three months of therapy with budesonide, a corticosteroid, increased the number of ciliated cells thereby increasing mucosal clearance more than bronchodilator treatment alone in patients with asthma. (5,7,8) Effect of Corticosteroids on Profibrotic Cytokines and VEGF in Severe Asthma Patients with moderate and severe asthma have increased amounts of the profibrotic cytokines IL-11, IL-17 and transforming growth factor beta (TGF-ß) when compared to healthy controls and patients with mild asthma. Treatment with two weeks of oral corticosteroids decreases the amounts of IL-11 and IL-17, but not of TGF-ß or collagen. (5) Treatment with corticosteroids affects the extracellular matrix components in asthma, but it is unclear whether this has functional relevance. Morphometric studies of bronchi have shown that patients with asthma have more blood vessels in both the large and small airways than patients without asthma.(5) This increase in the number of blood vessels is associated with increased microvascular permeability. Inhaled corticosteroids decrease airway vascularity in patients with asthma, which is associated with a decrease in basement membrane thickness, improvement in forced expiratory volume in one second (FEV1), and airway responsiveness. (7) Vascular endothelial growth factor (VEGF) is an important regulator of angiogenesis, and corticosteroids inhibit angiogenesis in part by regulating VEGF. (5,8) Beclomethasone 800 mcg/day decreases the levels of VEGF in induced sputum in asthma and is associated with a decrease of airway narrowing and vascular permeability. It appears that high doses of corticosteroids are necessary to reduce structural changes in airway vessels that are accompanied by decreases in VEGF expression. Fluticasone 700 mcg twice daily reduces the number of VEGF-positive vessels and VEGF receptors and decreases the concentration of angiopoietin 1. (5,8) Combining salmeterol, a ß2- agonist, with low-dose inhaled steroids decreases vessel density in patients with asthma, and ß2- agonists inhibit plasma exudation and vascular permeability. Results from a mouse model suggest that leukotriene receptor antagonists may decrease vascular permeability and VEGF levels. (7) Two weeks of daily treatment with 400 mcg fluticasone, a corticosteroid, was effective in reducing mucosal blood flow in patients with asthma. (5,7) Effect of Antigen Challenge and Proinflammatory Cytokines on Beta-Adrenergic Receptor Function Antigen challenge decreases the adenylate cyclase response and cyclic AMP levels in the T- lymphocytes of asthmatic patients. Antigen challenge in the sensitized guinea pig model decreases ß-adrenergic receptor-mediated relaxation in airway smooth muscle. This was attributed to the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and IL-1ß, which were also able to significantly reduce the responsiveness to isoproterenol. However, in these studies the contractile response of the trachea to carbachol was not increased (1,2,3). The in vitro response of trachea and airway smooth muscle to muscarinic agonist, such as acetylcholine, methacholine or carbachol, does not correlate with the in vivo airway hyperresponsiveness to methacholine as seen in patients with asthma, however the postulated mechanisms for this difference are controversial. Interestingly, decreased ß-adrenergic bronchodilator activity and associated hypersensitivity to mediators were theorized as potential pathophysiologic mechanisms several decades before the role of cytokines in asthma was known. (9) In an in vivo mouse model, treatment of nonsensitized mice with a combination of IL-1 and TNF-α significantly impaired the ability of ß2 agonists to prevent bronchial hyperresponsiveness (3). These results demonstrate that the proinflammatory cytokines, IL-1ß and TNF-α, attenuate bronchodilator responses to ß2 agonists by decreasing cyclic AMP production. Although TNF-α, IL-1ß and IL-13 are increased in the bronchi of asthmatic patients, the interrelationship of the effects of these cytokines is not clear. (2) IL-13 inhibits inflammation and the production of IL-1ß and TNF-α from monocytes and alveolar macrophages (1,3). Airway Smooth Muscle Proliferation and Airway Hyperresponsiveness: Role of Cytokines, Corticosteroids and Thermoplasty Increased airway smooth muscle due to hyperplasia and hypertrophy is presumed to be a major determinant of enhanced bronchoconstriction and airway hyperresponsiveness (AHR), (5,6) although the increase in proliferation of airway smooth muscle has not been confirmed in humans in vivo. Contractile agonists, cytokines, growth factors and extracellular matrix proteins can all contribute to airway smooth muscle proliferation. (5) Corticosteroids can exert a direct effect on airway smooth muscle cells in addition to modulating the secretion of chemokines and cytokines involved in airway smooth muscle proliferation. (7) In vitro studies demonstrate that corticosteroids arrest human airway smooth muscle cells in the G-1 phase of the cell cycle and inhibit some of the proliferation of airway smooth muscle cells induced by growth factors. There is some evidence that a combination of inhaled corticosteroids and ß2-adrenoceptor agonists is more efficacious than glucocorticoids alone in controlling remodeling events; (5) however, there are no longitudinal studies evaluating the effect of pharmacologic treatment on the structure of airway smooth muscle in asthma. Thermoplasty, which involves ablation of the airway smooth muscle, has been proposed as a treatment for asthma. Some evidence has indicated that bronchial thermoplasty reduces airway smooth muscle and increases airway compliance. The first (uncontrolled) study suggested that thermoplasty led to improvements in FEV1 and airway hyperresponsiveness. (5) Recently, the results of a controlled, randomized trial in patients with moderate to severe asthma indicated that thermoplasty reduces the number of mild exacerbations and improves morning expiratory peak flow and symptom scores, but has no effect on FEV1 and airway hyperresponsiveness after one year of follow-up. (5) Tumor necrosis factor α (TNF-α) and other inflammatory mediators of asthma are upregulated in patients with refractory asthma. This is the basis for treatment with anti-TNF-α antibodies. In two studies, etanercept, an anti-TNF-α antibody, decreased asthma symptoms and bronchial hyperresponsiveness and increased lung function. (10,11) Infliximab, which binds to and neutralizes TNF-α, decreases the number of moderate exacerbations and sputum cytokine levels, but does not affect lung function parameters. IL-4 and IL-5 antagonists, which may decrease markers of inflammation, have not been proven to improve airway hyperreactivity. IL-13 may be a more attractive target than TNF-α with regard to airway remodeling because it is present in the bronchial mucosa and sputum of asthmatics and is thought to have profibrotic activity. (1) IL-13-transgenic mice have markedly increased fibrosis, eosinophilia, airway hyperresponsiveness and goblet cell mucus production. (12) IL-13 knockout mice have reduced airway collagen, goblet cell metaplasia and mucus staining. Therefore, IL-13 is a reasonable target for preventing airway remodeling. Both IL-13 and the proinflammatory cytokines IL-1ß and TNF-α have been found in airway smooth muscle and are increased in the airway lining fluid of subjects with asthma. These cytokines decrease the relaxation response to ß-adrenergic agonists in airway smooth muscle. (1,3) However, IL-13 is both necessary and sufficient to produce the characteristics of asthma (1) and is a central mediator of allergic asthma. IL-13 elicits decreased adrenergic bronchodilator activity and associated hypersensitivity to mediators (9), thus supporting its role in the pathogenesis of asthma (2). Immunological and Clinical Changes in Allergic Asthmatics Following Treatment with Omalizumab Cytokines, including IL-13, also appear to contribute to allergic asthma. The anti-IgE antibody omalizumab reduces exacerbations and the requirement for steroids in allergic asthmatics. (13,14) This therapeutic effect of omalizumab can be at least partially attributed to the anti-inflammatory effect of decreasing IgE levels. Noga and colleagues examined whether treatment with omalizumab for 16 weeks leads to changes in inflammatory mediators and clinical symptoms in patients with moderate to severe allergic asthma. (15) They observed that omalizumab significantly decreased ß2-agonist use and wheal reactions to skin-prick tests. They also measured circulating levels of IL-5, IL-6, IL-8, IL-10, IL-13 and s-ICAM before and after 16 weeks of treatment with omallizumab. IL-13 decreased significantly in the omalizumab group compared to the placebo group, and IL-5 and IL-8 decreased in the omalizumab group compared to baseline. The levels of other circulating cytokines did not change. The release of histamine from blood basophils and the peripheral blood eosinophil count both decreased significantly. Because omalizumab binds free circulating IgE and prevents the interaction between IgE and its receptors on inflammatory cells (13), these studies point to IgE as a key mediator of allergic reactions in the upper and lower airways. (14) IL-13: A Cause of AHR in Asthma and Decreased Response to ß-adrenergic Agonists IL-13 induces AHR, IgE production, eosinophilia and goblet cell mucus production in the airways of mice. IL-13 produces AHR through its effect on the IL-4 receptor α chain and STAT-6, independently of IL-5 and eotaxin (1), and the blockade of IL-13 decreases the characteristic features of asthma in a mouse model of allergen sensitization and challenge(1). A single administration of exogenous IL-13Rα2 can inhibit the AHR induced by IL-13 by reducing the amount available to endogenous receptors (2,3). The use of IL-13R-α2 decoy receptors and an IL-13 mutant (IL-13E13K) are therefore potential approaches to the treatment of asthma (2). Ongoing clinical trials in patients with asthma include several IL-13 receptor antagonists as well as IL-13 monoclonal antibodies. Wenzel and colleagues reported that a monoclonal antibody that blocks the binding of IL-4 and IL-13 to IL-4 receptor complexes significantly inhibited the early and late reactions to allergen challenge in subjects with asthma (16). The development of allergen-induced airway hyperreactivity, the decreased response to ß-adrenergic agonists and the ability of IL-13 R-α2 to block this effect in mouse airway smooth muscle all support a critical role for IL-13 in asthma (1,2). In IL-13 knockout mice, ongoing challenge with allergen does not elicit AHR and airway mucus changes. Administration of recombinant IL-13 results in airway hyperresponsiveness and goblet cell mucus changes. (1, 17) .The initiation of TH2 immune responses to allergen is induced by IL-4; the main characteristics of asthma, such as AHR, airway fibrosis and mucus hypersecretion, are induced by IL-13 alone (1). IL-13 switches on the production of IgE and increases eosinophilic inflammation and mucus cell hyperplasia even in naïve, nonimmunized mice (1). In this regard, IL-13 is both necessary and sufficient to produce both characteristics of asthma (1). In addition, IL-13 markedly decreases the relaxation of mouse trachea in response to ß-agonists in vitro and also markedly diminishes the protective effect of albuterol in vivo against methacholine-induced bronchoconstriction (2). IL-13 has many diverse effects on a variety of cell types that are involved in the pathogenesis of allergic disorders (see table Functions of IL-13 on Inflammatory cells in Asthma). IL-13 induces VCAM-1 and enhances proliferation and cholinergic-induced contraction of airway smooth muscle cells in vitro.(1) IL-13 also increases collagen deposition and fibrosis. Furthermore it increases the expression of chemokines, including eotaxin and CCR5. (12) It is also a potent stimulator of matrix metalloproteinases in the lung. The expression of IL-13 (12) in transgenic mice results in emphysematous changes and mucus metaplasia. It therefore appears that IL-13 is an important molecule, not only in asthma, but also in chronic obstructive pulmonary disease phenotypes. (12) IL-13 and Corticosteroid-Resistant Asthma It was reported that the effect of fluticasone and/or ß-agonists on airway hyperresponsiveness in mice is markedly diminished through treatment with IL-13 (2, 4,18), both in vivo and in vitro. IL-13 also suppresses the airway response to glucocorticoids. During the preparation of this manuscript, another paper supporting the "finding" that IL-13 induces airway inflammation and a corticosteroid resistant model of severe asthma has been published. (19) Glucocorticoids remain the most effective therapy for asthma, primarily through their suppression of airway inflammation, but glucocorticoids do not affect airway hyperresponsiveness or the expression of goblet cell hyperplasia induced by IL-13. Corticosteroids inhibit IL-13 production by mast cells and peripheral blood mononuclear cells (i.e., TH2 cells) but have little or no effect on IL-13 once it is released. When IL-13 was administered to nonsensitized mice, administration of fluticasone by inhalation did not protect against methacholine bronchial challenges. In contrast, fluticasone and other corticosteroids were effective in protecting against AHR in ovalbumin-sensitized mice (2,4, 18,19). Table. Functions of Interleukin-13 (IL-13) on Inflammatory Cells in Asthma Target Tissues/cells Effects of IL-13 Respiratory epithelium Increases chemokine expression, mucus hypersecretion, and goblet cell metaplasia Airway smooth muscle Increases smooth muscle proliferation Increases sensitivity to bronchoconstrictor agents B-lymphocyte Induces immunoglobulin (Ig)E production In macrophages, increases low-affinity IgE receptors In mast cells, modulates the high-affinity IgE receptor and IgE priming Upregulates the IgE receptor Eosinophils Recruits and activates Increases the numbers of eosinophils Vascular endothelium Induces the expression of vascular cell adhesion molecules Increases chemokine expression, e.g. CCR5 Fibroblasts Increases collagen and fibrosis remodeling in airways? E.g. via TGF-ß Other mechanisms by which IL-13 induces the features of asthma have been summarized by Wills-Karp (1), including signaling through the adenosine, acidic mammalian chitinase, leukotriene and arginase signaling cascades (1). During the pathogenesis of asthma (3), IL-13 can elicit the loss of adrenergic bronchodilator activity associated with hypersensitivity to mediators, as put forth by Szentivanyi 40 years ago. (9,2) IL-13 thus affects a variety of genes in the cells of the airways, including smooth muscle cells, epithelial and endothelial cells, goblet cells, fibroblasts and monocytes, macrophages, B-cells, basophils and mast cells and eosinophils. The role of the chemokine CCR5 in the pathogenesis of IL-13 -induced inflammation and remodeling has been reported.(12) (See figure) Summary The pathogenesis of asthma involves inflammatory processes that result in structural airway changes and remodeling. The effect of the proinflammatory cytokines TNF-α and IL-1ß and especially IL-13 reduce the effect of endogenous and exogenous bronchodilators. The striking effect of IL-13 on rapidly inducing AHR and suppressing of the effect of corticosteroids emphasizes the importance of clinical trials of IL-13 or IL-13 receptors antagonists in asthma that are currently underway. References Wills-Karp M.: "Interleukin-13 in Asthma Pathogenesis". Immunol Rev 2004;202:175- 190. Townley R.: "IL-13 and ?-adrenergic Blockade Theory of Asthma Revisited 40 Years Later". Ann Allergy Asthma Immunol 2007; 99 (3):215-224. Townley RG, Horiba M. "Airway Hyperresponsiveness: Story of Mice and Men and Cytokines". Clin Rev Allergy Immunol 2003;24 (1):85-110. Townley R G, Gendapodi P, Romero FA "Effect of Fluticasone and/or ?-agonists on Airway Hyperresponsiveness (AHR) in Mice Either Sensitized to Allergen or Pre-Treated with IL-13". J Allergy Clin Immunol 2007;119:S295. (abstract) Mauad T, Bel EH, Sterk PJ: "Asthma Therapy and Airway Remodeling". J Allergy Clin Immunol 2007; 120:997-1009. Barnes, P.J.: Pathophysioslogy of Allergic Inflammation. Chapter 30, p. 483, Middleton 6th Ed. Allergy Principles and Practices, 2003, Mosby, St. Louis, MO. Schleimer R. "Glucocorticoids Chapter 52, p. 870-877 in Middleton, Reed & Ellis, Allergy Principles and Practice, 6th Edition 2003; Mosby St Louis, MO. Niazi S, Bata V, Awsare B, Zangrilli J, Peters SP, Chapter 28 p.453- 461: Allergic Inflammation and Initiation, Progression and Resolution. In Middleton, Reed & Ellis, 6th Edition Allergy, Principles and Practice 2003; Mosby, St Louis, Mo. Szentivanyi A: ‚ The ß-adrenergic theory of the atopic abnormality in bonchial asthma. J Allergy 1968;42:203-233. Erin E, Leaker BR, Nicholson GC, Tan AJ, Green, LM , Neighbour H, Zacharasiewicz AS, Turner J, Barnathan ES, Kon OM, Barnes PJ,Hansel TH.: The Effects of a Monoclonal Antibody Directed against Tumor Necrosis Factor- in Asthma Am. J. Respir. Crit. Care Med. 2006; 174: 753-762. Berry MA, et al: Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med 2006; 354(7):697-708. Ma B, Liu W, Homer RJ, Lee PJ, Coyle AJ, Lora JM, Lee CG, Elias JA. Role of CCR5 in the Pathogenesis of IL-13-Induced Inflammation and Remodeling. J Immunol 2006 176: 4968-4978. Soler M, Matz J, Townley R, Buhl R, O'Brien J, Fox H et al. The anti-IgE antibody Omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J 2001; 18(2): 254-61. Stokes J, Casale TB. Rationale for new treatments aimed at IgE immunomodulation. Ann Allergy Asthma Immunol. 2004 Sep;93(3):212-7; quiz 217-9, 271. Noga,O, Hanf G, Kunkel G.: "Immunological and Clinical Changes in Allergic Asthmatics Following Treatment with Omalizumab". Int Arch Allergy Immunol 2003;131:46-52. Wenzel S, Wilbraham D, Fuller R, Getz, EB, Longphre M: "Effect of an Interleukin-4 Variant on Late Phase Asthmatic Response to Allergen Challenge in Asthmatic Patients: Results of Two Phase II Studies". Lancet, 2007;370(9596):1396-8. Zhu Z, Ma B, Zheng T, Homer RJ, Lee CG, Charo IF, Noble P, Elias JA.: IL-13 transgenic mice. IL-13-Induced Chemokine Responses in the Lung: Role of CCR2 in the Pathogenesis of IL-13-Induced Inflammation and Remodeling. J Immunol 2002 168: 2953-2962. Townley RG, Gendapodi PR, Romero FA, Qutna N, Abel P.: IL-13 Induces Bronchial Hyperresponsiveness and Decreases the Bronchoprotective Effect of Beta-Adrenergic Bronchodilators and Corticosteroids. Ann allergy Asthma & Immunol (in press) Therien AG, Bernier V, Weicker S, Tawa P, Falgueyret JP, Mathieu MC, Honsberger J, Pomerleau V, Robichaud A, Stocco R, Dufresne L, Houshyar H, Lafleur J, Ramachandran C, O'Neill GP, Slipetz D, Tan CM. Adenovirus IL-13-induced airway disease in mice: a corticosteroid-resistant model of severe asthma. Am J Respir Cell Mol Biol. 2008 Jul; 39(1):26-35.

|
Scooped by
Gilbert C FAURE
March 7, 2019 6:34 AM
|

|
Scooped by
Gilbert C FAURE
March 4, 2019 1:45 PM
|
We are a European alliance of 41 allergy, asthma and chronic obstructive pulmonary disease (COPD) patients’ associations representing 30% of European citizens currently living with these diseases. Our mission is to convey their voice and to be actively involved in the decisions impacting their...

|
Suggested by
Société Francaise d'Immunologie
February 20, 2019 4:02 AM
|
Abstract A 60‐year‐old female with severe bronchial asthma developed persistent dyspnoea and an abnormal lung shadow. High‐resolution computed tomography (HRCT) demonstrated patchy ground‐glass opacities and diffuse, small nodular shadows. Elevated percentages of eosinophils were observed in the blood and bronchoalveolar lavage fluid. These results collectively indicated that her asthma was accompanied by eosinophilic pneumonia and eosinophilic bronchiolitis. Although previous, rare case reports suggest that systemic steroid therapy is necessary and effective for the control of eosinophilic bronchiolitis, we chose to treat her with an anti‐interleukin 5 antibody, mepolizumab. Her asthma, eosinophilic pneumonia, and eosinophilic bronchiolitis each improved in response to mepolizumab as assessed from her symptoms, pulmonary function tests, and HRCT. Mepolizumab might be effective not only for asthma and eosinophilic pneumonia but also for eosinophilic bronchiolitis. Introduction There have been a few case reports of eosinophilic bronchiolitis that is characterized by radiographic findings showing diffuse bronchiolitis plus massive accumulation of eosinophils in the airways 1-4. It has been suggested that systemic steroid therapy is effective for this disorder, although the precise pathogenesis in unknown. Here, we report a case of severe asthma complicated with eosinophilic pneumonia and eosinophilic bronchiolitis, all of which were alleviated by anti‐interleukin 5 (IL‐5) antibody. Case Report A 60‐year‐old female was referred to the outpatient clinic of Teikyo University Hospital for evaluation of persistent dyspnoea and an abnormal lung shadow. She had been diagnosed with bronchial asthma 10 years earlier. Although she continued to use medium‐ or high‐dose inhaled corticosteroid (ICS) plus a long‐actingβ2 agonist (LABA), leukotriene receptor antagonist (LTRA), and sustained‐release theophylline, the asthma frequently flared up, and short‐term oral corticosteroid bursts were needed. One year before referral, diffuse small nodular shadows were seen on chest X‐rays, suggesting the possibility of bronchiolitis. On the day of her first visit to our clinic, she complained of persistent dyspnoea both at rest and on exertion and presented expiratory wheezes. Her fractional exhaled nitric oxide (FeNO) was clearly elevated (72 ppb). Blood tests showed eosinophilia (790/μL), elevated serum total IgE (1280 IU/mL), and positivity for specific IgEs against house dust mites and aspergillus. Serum autoantibodies, including myeloperoxidase–anti‐neutrophil cytoplasmic antibody (ANCA) and proteinase 3‐ANCA, were negative. A chest X‐ray showed diffuse small nodular shadows and irregular pulmonary infiltration shadows (Fig. 1A). High‐resolution computed tomography (HRCT) images demonstrated a tree‐in‐bud appearance and patchy ground‐glass opacity (GGO) in both lung fields (Fig. 1B), suggesting the presence of bronchiolitis and pneumonia. Sinus computed tomography (CT) showed non‐specific mild maxillary sinusitis but no ethmoid sinusitis. After starting inhalation of tiotropium, a muscarinic antagonist, her asthma symptoms improved slightly, although a pulmonary function test clearly indicated airflow obstruction (Fig. 1C). Bronchoscopic examination found that the bronchial mucosa was oedematous, and the bronchoalveolar lavage (BAL) fluid showed an elevated percentage of eosinophils (28.5%) but not neutrophils. A biopsy specimen of the right B8 distal bronchial mucosa showed massive infiltration of eosinophils, detachment of airway epithelial cells, and thickening of subepithelial fibrosis, but no Charcot‐Leyden crystals were observed (Fig. 1D). These findings resulted in a diagnosis of bronchial asthma, eosinophilic pneumonia, and eosinophilic bronchiolitis. As her symptoms persisted, we decided to start treatment with mepolizumab, an anti‐IL‐5 antibody, two months after her first visit to our hospital. Her dyspnoea gradually improved, and her blood eosinophil counts were controlled at low levels, although FeNO remained high (Fig. 2A). HRCT images indicated that GGO had disappeared, and the thickening of the bronchial mucosa observed in the initial HRCT images had become milder (Fig. 2B, C). The tree‐in‐bud appearance and thickening of centrilobular shadows, suggesting bronchiolitis, were also alleviated. A spirogram showed improvement in both restrictive abnormality (percent vital capacity (%VC): 70.9% before mepolizumab; 94.8% after introduction of mepolizumab) and obstructive impairment (forced expiratory volume in 1 second (FEV1): 0.99 L before mepolizumab; 1.45 L after introduction of mepolizumab) (Fig. 2A). The residual volume/total lung capacity (RV/TLC), a useful index of air trapping in relation to small airway involvement, was initially as high as 46.9% (two months after mepolizumab was started), but it gradually improved with time to 44.4% (after four months on mepolizumab) and then 40.4% (after 10 months).Oral steroid bursts were not necessary during treatment with mepolizumab. Discussion Eosinophilic bronchiolitis is a relatively new disorder, first reported in 2001 1. So far, around 10 cases of this disorder have been reported; all of them displayed chronic progression of respiratory symptoms including cough, sputa, and dyspnoea at rest and exertion. This disorder is characterized by unique radiological findings, i.e. diffuse micronodular shadows and a tree‐in‐bud appearance, suggesting bronchiolitis and eosinophilia in both blood and pulmonary examinations 1-4. The clinical features of our case are in line with those findings for eosinophilic bronchiolitis. Thus, we believe that the diagnosis of eosinophilic bronchiolitis is correct for the present case. Accumulating evidence suggests that eosinophilic bronchiolitis is often accompanied by various other eosinophilic disorders 2, 5. Bronchial asthma is the most commonly reported disease accompanying eosinophilic bronchiolitis, as seen in our case, who also had eosinophilic pneumonia. In this patient, the findings of diffuse bronchiolitis on CT images were dominant and very striking, and we felt that they could not be regarded as features of bronchial asthma. The patient was thus diagnosed with a combination of asthma and eosinophilic bronchiolitis. We suppose that her asthma, eosinophilic pneumonia, and eosinophilic bronchiolitis might be mutually related, and these disorders collectively gave rise to cough, dyspnoea, and clear impairment of pulmonary function. It is important to note that BAL analysis may not be useful for distinguishing eosinophilic bronchiolitis as other disorders also demonstrate a similar increase in eosinophils. The previous case reports on eosinophilic bronchiolitis suggested that oral corticosteroid was an effective and standard therapy, whereas ICS was not. Importantly, discontinuation of oral corticosteroid was difficult, and long‐term administration of systemic steroid was thus unavoidable 1, 3, 4. For our patient, however, we chose a new anti‐IL‐5 antibody, mepolizumab, as her asthma was severe, and she strongly requested an additional effective anti‐asthma drug other than systemic steroid. As a result, not only her asthma but also her eosinophilic pneumonia and eosinophilic bronchiolitis responded to mepolizumab: her symptoms improved, as did the findings of lung function and imaging studies. Her clinical course suggests that IL‐5 may have been critically involved in the pathogenesis of all of her eosinophilic disorders, including eosinophilic bronchiolitis. As there have not been many reports of eosinophilic bronchiolitis, we have limited evidence regarding the pathogenesis and standard therapy for this disorder. In view of recent robust progress in the field of allergology, further accumulation of basic and clinical information on eosinophilic bronchiolitis is anticipated. That information will contribute to the further confirmation of this clinical entity, e.g. whether eosinophilic bronchiolitis is a unique disorder or just a continuum of the pathological process of asthma and the therapeutic strategy for it, and to our overall understanding of eosinophilic lung diseases. Disclosure Statement Appropriate written informed consent was obtained for publication of this case report and accompanying images. Acknowledgments We thank Ms Yasuko Asada for her excellent secretarial work. References

|
Scooped by
Gilbert C FAURE
February 6, 2019 9:00 AM
|
|



 Your new post is loading...
Your new post is loading...



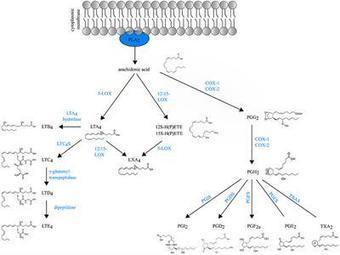


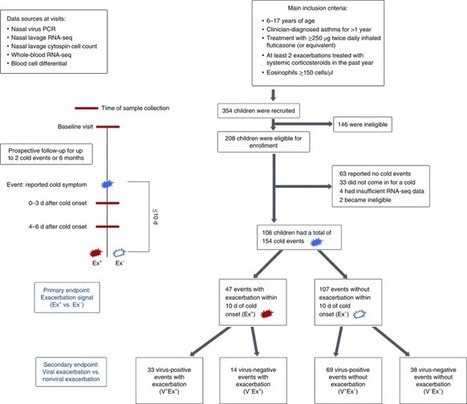







![[EP Interest Group on Allergy and Asthma] Championing a renewed EU agenda on allergy and airways diseases for a healthier Europe | Allergy (and clinical immunology) | Scoop.it](https://img.scoop.it/QCSoasgKe_ZwqM5L2N6uGzl72eJkfbmt4t8yenImKBVvK0kTmF0xjctABnaLJIm9)





