Your new post is loading...

|
Scooped by
Juan Lama
June 24, 10:48 AM
|
RetroVirox has launched a Summer Promotion with a 30% discount for antiviral and neutralization services against 4 viruses, including influenza, dengue, human metapneumovirus (HMPV) and respiratory syncytial virus (RSV). Discount applies to services initiated between July 1 and August 31 2025
Contact us at info@retrovirox.com for inquiries and additional info

|
Scooped by
Juan Lama
October 29, 10:51 AM
|
Since 2020, high pathogenicity avian influenza virus (HPAIV) infections in wild birds have been frequently reported. Because HPAIV infection has occasionally caused outbreaks in captive rare birds, application of antiviral drugs for treatment purposes against them has been considered from the perspective of conservation medicine. In this study, the therapeutic efficacy of baloxavir marboxil (BXM) was evaluated using a duck model to help establish the post-infection treatment for rare birds. Sixteen four-week-old ducks were divided into four groups and intranasally inoculated with the HPAIV strain A/crow/Hokkaido/0103B065/2022 (H5N1). BXM was orally administered once daily at doses of 12.5, 2.5, 0.5, and 0 mg/kg to each of the four groups from 2 to 6 days post-infection. Blood samples were collected at 2, 8, and 24 hours after the initial BXM administration to measure the plasma concentrations of its active form, baloxavir acid (BXA). All ducks were monitored until 14 days post-infection, and their oral and cloacal swabs were collected for virus recovery. All eight ducks administered with 12.5 or 2.5 mg/kg of BXM survived, demonstrating a significant reduction in virus recovery compared to the 0 mg/kg group. Pharmacokinetic/pharmacodynamic (PK/PD) analysis of BXA suggested that parameters such as Cmax and AUC0–24hr were correlated with the suppression of virus shedding. These findings demonstrated that BXM administration within 48 hours post-HPAIV infection in ducks effectively reduced mortality and virus shedding. The comparison of PK parameters may help estimate efficient BXM dosing strategies in rare birds. Preprint in medRxiv (October 28, 2025): https://doi.org/10.1101/2025.10.24.684283

|
Scooped by
Juan Lama
October 27, 12:31 PM
|
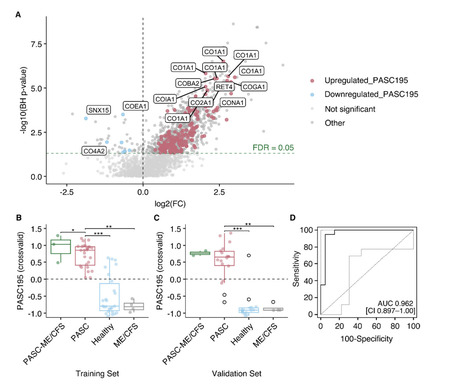
Background Post-acute sequelae of severe acute respiratory syndrome coronavirus 2-infection (PASC) is challenging to diagnose and treat, and its molecular pathophysiology remains unclear. Urinary peptidomics can provide valuable information on urine peptides that may enable improved and specified PASC diagnosis. Methods Using standardized capillary electrophoresis-MS, we examined the urinary peptidomes of 50 patients with PASC 10 months after COVID-19 and 50 controls including healthy individuals (n = 42) and patients with non-COVID-19-associated myalgic encephalomyelitis/chronic fatigue syndrome (n = 8). Based on peptide abundance differences between cases and controls, we developed a diagnostic model using a support vector machine. Results The abundance of 195 urine peptides among PASC patients significantly differed from that in controls, with a predominant abundance of collagen alpha chains. This molecular signature (PASC195), effectively distinguished PASC cases from controls in the training set [AUC of 0.949 (95% CI 0.900–0.998; p < 0.0001)] and independent validation set [AUC of 0.962 (95% CI 0.897–1.00); p < 0.0001)]. In silico assessment suggested exercise, GLP1-RA and MRA as potentially efficacious interventions. Conclusions We present a novel and non-invasive diagnostic model for PASC. Reflecting its molecular pathophysiology, PASC195 has the potential to advance diagnostics and inform therapeutic interventions. Statement of significance of the study Despite the recent emergence of omics-derived candidates for post-acute sequelae of SARS-CoV-2 infection (PASC), the pending validation of proposed markers and lack of consensus result in the continuous reliance on symptom-based criteria, being subject to diagnostic uncertainties and potential recall bias. Building upon prior findings of renal involvement in acute COVID-19 pathophysiology and PASC-associated alterations, we hypothesized that the use of urinary peptides for PASC-specific biomarker discovery, unlike conventional specimens that have been utilized thus far, may offer complementary information on putative disease mechanisms. In the present study, 195 significantly expressed peptides were used to form a classifier termed PASC195, which effectively discriminated PASC from non-PASC (p < 0.0001), including healthy individuals and non-COVID-19 associated myalgic encephalomyelitis/chronic fatigue syndrome, in both the derivation (n = 60) and an independent validation set (n = 40). Shift in collagen regulation was associated with PASC, as the majority of PASC195 peptides were derived from collagen alpha chains. Ongoing inflammatory responses, hemostatic imbalances, and endothelial damage were inferred from cross-sectional variations in endogenous peptide excretion. Available in medRxiv (October 27, 2025): https://doi.org/10.1101/2025.10.15.25338065

|
Scooped by
Juan Lama
October 24, 11:53 AM
|
SARS-CoV-2 has been shown to induce autoimmunity. Due to the idiotype-antiidiotypic interactions between lymphocytes to SARS-CoV-2 S protein and lymphocytes to angiotensin converting enzyme 2 (ACE2), the immune response to S protein can cause induction of anti-ACE2 lymphocytes. The ubiquity of ACE2 within the human body endows autoimmune reactions to ACE2 with the role of the main factor causing injuries of various tissues. Immunization of Wistar rats with S protein caused hyperplasia and dedifferentiation of type II pneumocytes, extensive injury of the proximal tubules, infiltration of CD4+ T, CD45RA+ B lymphocytes in the lungs and CD4+ T, CD8+ T lymphocytes in the kidneys. Both type II pneumocytes and proximal tubule epithelium express ACE2. Damage to ACE2 expressing cells in the absence of other lesions in the studied organs suggests that ACE2 might be the target of an autoimmune attack induced by S protein. Our findings clarify the mechanism of multiple tissue damage in COVID-19. Published (Oct. 24, 2025) in Scientific Reports: https://doi.org/10.1038/s41598-025-21304-y

|
Scooped by
Juan Lama
October 21, 12:30 PM
|
Recognition of foreign RNA is critical for the innate immune response to viruses. Interferon (IFN)-induced proteins with tetratricopeptide repeats (IFIT) 2 and 3 are highly upregulated following viral infection, but mechanistic insight into their antiviral role is lacking. Here we demonstrate that short 5’ untranslated regions (UTRs), a characteristic of many viral mRNAs, can serve as a molecular pattern for innate immune recognition via IFIT2 and IFIT3. Structure determination of the IFIT2–IFIT3 complex at 3.2 Å using cryo-EM reveals a domain-swapped heterodimer that is required for recognition of the viral mRNA 5’ end, translation inhibition and antiviral activity. Critically, viral or host 5’ UTR lengths less than 50 nucleotides are necessary and sufficient to enable translation inhibition by the IFIT2–IFIT3 complex. Accordingly, diverse viruses whose mRNAs contain short 5’ UTRs, such as vesicular stomatitis virus and parainfluenza virus 3, are sensitive to IFIT2–IFIT3-mediated antiviral activity. Our work thus reveals a pattern of antiviral nucleic acid immune recognition that takes advantage of the inherent constraints on viral genome size. Viruses generally have compact genomes, resulting in many viral mRNAs with short 5’ untranslated regions. An antiviral complex exploits this feature of viral mRNAs to selectively inhibit viral protein synthesis and repress viral replication. Published in Nature Microbiology (Oct. 15, 2025): https://doi.org/10.1038/s41564-025-02138-w

|
Scooped by
Juan Lama
October 19, 11:55 AM
|
Background Ensitrelvir is an oral antiviral treatment for COVID-19 with the same molecular target (the main protease) as ritonavir-boosted nirmatrelvir—the current oral first-line treatment. We aimed to compare the clinical antiviral effects of the two drugs. Methods In an open-label, phase 2, randomised, controlled, adaptive pharmacometric platform trial, low-risk adult outpatients aged 18–60 years with early symptomatic COVID-19 (<4 days of symptoms) were recruited from hospital acute respiratory infection clinics in Thailand and Laos. Patients were randomly assigned in blocks (block sizes depended on the number of interventions available) to one of eight treatment groups, including oral ensitrelvir and oral ritonavir-boosted nirmatrelvir at standard doses, both given for 5 days, and no study drug. The primary endpoint was the oropharyngeal SARS-CoV-2 viral clearance rate assessed between day 0 and day 5 in the modified intention-to-treat population (defined as patients with at least 2 days of follow-up). Patients had four oropharyngeal swabs taken on day 0 and two swabs taken daily from days 1 to 7, then on days 10 and 14. Viral clearance rates were derived under a Bayesian hierarchical linear model fitted to log10 viral densities in standardised paired oropharyngeal swab eluates taken daily over the 5 days (14 samples). An individual patient data meta-analysis of all small molecule drugs evaluated in this platform trial using published results was also performed, adjusting for temporal trends in viral clearance. This trial is registered at ClinicalTrials.gov, NCT05041907. Findings Between March 17, 2023, and April 21, 2024, 604 of 903 patients enrolled were concurrently assigned to the three treatment groups (ensitrelvir n=202; ritonavir-boosted nirmatrelvir n=207; no study drug n=195). Median estimated SARS-CoV-2 clearance half-lives were 5·9 h (IQR 4·0–8·6) with ensitrelvir, 5·2 h (3·8–6·6) with nirmatrelvir, and 11·6 h (8·1–14·5) with no study drug. Viral clearance following ensitrelvir was 82% faster (95% credible interval 61–104) than no study drug and 16% slower (5–25) than ritonavir-boosted nirmatrelvir. In the meta-analysis of all unblinded small molecule drugs evaluated in the platform trial, nirmatrelvir and ensitrelvir had the largest antiviral effects (1157 patients). Viral rebound occurred in 15 (7%) of 207 patients in the nirmatrelvir group and 10 (5%) of 202 in the ensitrelvir group (p=0·45). Interpretation Both ensitrelvir and nirmatrelvir accelerate oropharyngeal SARS-CoV-2 viral clearance. Ensitrelvir is an effective alternative to currently available antivirals in treating COVID-19. Although COVID-19 is now generally a mild disease, it still causes substantial morbidity, particularly in vulnerable groups, and new variants or other coronaviruses could still emerge with pandemic potential. Safe effective and affordable antivirals are needed, and these are best assessed initially in pharmacometric platform trials assessing viral clearance. Published in The Lancet Infectious Diseases (October 10, 2025)

|
Scooped by
Juan Lama
October 16, 10:56 AM
|
In a groundbreaking development that promises to reshape the landscape of influenza vaccination, researchers have unveiled a novel vaccine that occupies a central position within the antigenic space of the A(H5) influenza virus. This innovative vaccine formulation is engineered to confer broad immunity across diverse strains of the highly pathogenic avian influenza H5 subtype, potentially offering a crucial tool in the ongoing battle against the threat posed by influenza zoonosis and pandemics. The meticulous phylogenetic analyses that underpin this advancement were enabled by leveraging comprehensive HA nucleotide sequences sourced from extensive global databases, ensuring the vaccine’s design harmonizes with the genetic diversity of circulating H5 viruses. Central to this achievement is the integration of an unparalleled dataset comprising nearly 15,000 hemagglutinin sequences from numerous H5 isolates worldwide. By employing sophisticated bioinformatics pipelines—including sequence deduplication, alignment via advanced algorithms like MAFFT, and maximum-likelihood phylogenetic tree construction via IQ-Tree2—the team systematically delineated the antigenic relationships within the H5 subtype. This approach, combined with cutting-edge computational tools for clade prediction, allowed researchers to pinpoint a vaccine candidate uniquely positioned to bridge antigenic variations across clades, enhancing cross-protective potential. The cultivation and maintenance of requisite cellular models such as 293T and MDCK cells supported the intricate manipulations necessary for recombinant virus production. Optimized culture conditions maintained under precisely controlled environments ensured high-fidelity replication of viral vectors employed in the reverse genetics system. The rigorous plasmid construction process involved cloning HA and NA gene segments sourced from in-house viral isolates or synthesized genes with strategically altered cleavage sites, highlighting the ingenuity in viral engineering designed to attenuate pathogenicity while preserving immunogenic features. An essential ethical and collaborative aspect of the research was the adherence to equitable benefit-sharing agreements facilitated through GISAID. By committing to openly share synthetic constructs, recombinant viruses, and ferret sera with all contributing laboratories globally, the researchers fostered an ecosystem of transparency and reciprocity critical during a time when global health challenges necessitate unified scientific responses. This commitment underscores the role of international cooperation in accelerating the translation of genomic data into actionable vaccine solutions. Strict biosafety protocols underscored every aspect of the research. Work involving recombinant viruses harboring attenuating mutations was conducted under BSL2 conditions, whereas wild-type highly pathogenic avian influenza isolates received handling within BSL3 and ABSL3+ facilities. Such meticulous adherence to biosafety practices ensured the containment of infectious agents while enabling experimental progression, particularly in ferret challenge studies that evaluated vaccine efficacy under realistic infection scenarios. The generation of recombinant influenza viruses through reverse genetics was pivotal to vaccine development, necessitating precise transfections and virus propagation in both cell culture and embryonated egg systems. Virus titration methods employing MDCK cells and hemagglutination assays provided robust measures of viral infectivity and antigen content, facilitating rigorous standardization of vaccine stocks. Sequencing confirmation of viral segments ensured genetic fidelity of constructs, crucial for reproducibility and safety. Vaccine production hinged on advanced purification techniques that enriched viral antigens whilst eliminating extraneous components. Through ultracentrifugation with sucrose gradients and subsequent solubilization, researchers isolated and processed whole-inactivated and split-inactivated vaccines that retained native antigenic configurations critical for eliciting effective immune responses. The quantification of hemagglutinin content employed mass spectrometry with stable isotope-labeled peptides, a gold-standard approach ensuring precise antigen dosing vital for immunogenic consistency. The experimental design extended to animal models, specifically ferrets, which serve as the gold standard for assessing influenza vaccine performance due to their physiologic and immunologic similarity to humans in respiratory viral infections. Through carefully controlled prime-boost vaccination regimens and subsequent challenges with wild-type recombinant viruses, the study generated comprehensive data on immune protection, viral shedding, and pathogenesis. The deployment of implanted temperature loggers and standardized clinical scoring permitted detailed monitoring of disease progression and vaccine efficacy, with downstream histopathological and immunohistochemical analyses elucidating the interplay between viral replication and host immune response. Serological assays, including hemagglutination inhibition and virus neutralization tests, constituted the cornerstone for gauging antibody-mediated immunity. These assays, performed with stringent controls and blinded assessments, quantified functional antibody titers against a panel of recombinant and wild-type viruses, thereby mapping the breadth of vaccine-induced protection. The precision of these assays underpins the robust correlates of immunity necessary for licensure pathways and public health application. Beyond conventional methods, the research leveraged antigenic cartography—a powerful computational technique that translates serological data into multidimensional spatial maps—to visualize and interpret the antigenic relationships among H5 viruses and vaccine-induced sera. This approach illuminates how vaccination shifts the humoral immune landscape, providing insights into antigenic drift, vaccine coverage, and potential gaps in immunity. Such granularity is instrumental in preemptively guiding vaccine strain updates in response to viral evolution. In a nuanced exploration of viral receptor specificity, the research incorporated assays using resialylated turkey red blood cells engineered to express distinct sialic acid linkages. This enabled precise characterization of viral hemagglutinin binding preferences, information pivotal for understanding host range and transmission potential. Validation using control viruses ensured assay fidelity, thereby solidifying conclusions on the biological behavior of vaccine strains. The data visualization and statistical analyses underpinning the study were executed with state-of-the-art bioinformatics tools and rigorous statistical frameworks. Employing packages within the R environment for visualization and hypothesis testing, the study provided transparent and reproducible analytic workflows. Statistical stringency through non-parametric tests and correction for multiple comparisons further assured the reliability of findings, reinforcing confidence in the vaccine’s broad immunogenicity claim. Collectively, this research represents a tour de force in influenza vaccinology, combining genomics, virology, immunology, and computational biology to deliver a vaccine candidate strategically situated at the heart of H5 antigenic diversity. Its broad immune coverage portends a significant advance in pandemic preparedness, particularly given the propensity of H5 viruses to undergo antigenic shifts that challenge existing vaccine paradigms. The methodologies and collaborative ethos exemplified herein herald a new era wherein vaccine design is as much a product of bioinformatics and global data sharing as it is of traditional virological expertise. Research published in Nature (Oct. 15, 2025): https://doi.org/10.1038/s41586-025-09626-3

|
Scooped by
Juan Lama
October 9, 10:35 AM
|
Regulatory T cells, which help to dampen inflammation, are being used in clinical trials against ailments such as rheumatoid arthritis. The class of immune cells at the centre of Monday’s Nobel prize is showing promise as a treatment for autoimmune diseases, cancer and even organ transplants — but there are still key challenges to overcome before these cells can be used in therapies in the clinic. Regulatory T cells, or Treg cells, help to prevent the body from attacking its own tissues. Earlier this week, the Nobel Prize in Physiology or Medicine went to three scientists who discovered Treg cells and helped to show how these cells regulate the immune system: immunologist Shimon Sakaguchi at the University of Osaka in Suita, Japan, Mary Brunkow, a molecular biologist at the Institute for Systems Biology in Seattle, Washington, and scientific adviser Fred Ramsdell, at the firm Sonoma Biotherapeutics in San Francisco, California. Thirty years after Sakaguchi and his colleagues reported the discovery of Treg cells, there are more than 200 clinical trials of the cells under way, for conditions such as type 1 diabetes, motor neuron disease and a host of autoimmune diseases — including multiple sclerosis, lupus and a group of rare skin disorders called pemphigus. The cells are also being tested for their ability to keep the immune system from rejecting transplanted tissues. “There’s a lot of excitement about recruiting regulatory T cell activity to treat a range of autoimmune or inflammatory disorders, but also in the context of tissue and cell transplants,” says Daniel Gray, a Treg-cell researcher at the Walter and Eliza Hall Institute of Medical Research in Parkville, Australia. But the rarity and fragility of these cells pose difficulties to their widespread application, researchers say. Finding the balance Autoimmune diseases are characterized by an imbalance of T cells: people with these conditions have more T cells that promote inflammation and kill disease-causing cells than T cells that reduce inflammation, says Joshua Ooi, who studies Treg-cell therapies for lupus at Monash University in Melbourne, Australia. “We can correct the balance by increasing the numbers of regulatory T cells,” he adds, which can help the body to suppress the pro-inflammatory T cells. Two methods to increase the number of Treg cells a person has are gaining ground, Ooi says. The first involves isolating a person’s Treg cells, growing more of them in the laboratory and infusing them back into the individual. The other method involves injecting people with drugs that trigger the body to make more Treg cells. Because Treg cells can control an overactive immune system, scientists are investigating them as a therapy to counter the side effects of bone marrow transplants, which involves building a new immune system in people with blood cancers, says Andrea Henden, a clinician–researcher at the QIMR Berghofer Medical Research Institute in Herston, Australia. Sonoma Biotherapeutics, of which Ramsdell was a co-founder, is operating clinical trials of modified Treg cells for rheumatoid arthritis and hidradenitis suppurativa — a chronic condition that causes painful bumps under the skin. Obstacles ahead There are significant challenges to the widespread deployment of Treg cells as a therapy. One of the key issues is that these cells are rare, making up only a small proportion of the T cells in the body, says Ooi. “Often when we isolate these anti-inflammatory regulatory T cells, they can be contaminated,” he says. “Or they may also contain some pro-inflammatory cells. The cells’ rarity makes it a challenge for researchers to collect them from a person and grow more of them in the lab at a high enough quantity to treat disease effectively, Henden says. “And then they do tend, when you culture them in a lab, to want to revert to a more regular cell type,” she adds, meaning that they are no longer useful for treatment. Gray says even if infusions contain lots of Treg cells, most of the cells die quickly because of bodily processes that work to maintain a certain number of these cells. “If we can overcome those processes, we’ll be able to sustain regulatory T cells” that have been infused into an individual to try and treat autoimmune diseases, he adds. It is also tricky to coax T cells to travel to the parts of the body in which they are needed. Researchers are currently working to modify Treg cells, giving them receptors that cause the cells to home in on the location of a tissue graft or an area in which there is active disease. If these challenges can be addressed, Gray says he is confident that Treg-cell therapies will become much closer to being used in the clinic. Ooi predicts that some treatments will enter the clinic in the next few years. “These regulatory cells, in theory, could be used to treat almost anyone with an autoimmune disease,” Ooi adds.

|
Scooped by
Juan Lama
October 8, 11:41 AM
|
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for the COVID-19 pandemic, utilizes membrane-bound, angiotensin-converting enzyme II (ACE2) for internalization and infection. We describe the development of a biologic that takes advantage of the proximity of the N-terminus of bound ACE2 to the three-fold symmetry axis of the spike protein to create an ultrapotent, trivalent ACE2 entry antagonist. Distinct disulfide bonds were added to enhance serum stability and a single point mutation was introduced to eliminate enzymatic activity. Through surface plasmon resonance, pseudovirus neutralization assays, and single-particle cryo-electron microscopy, we show this antagonist binds to and inhibits SARS-CoV-2 variants. We further show the antagonist binds to and inhibits a 2003 SARS-CoV-1 strain. Collectively, structural insight has allowed us to design a universal trivalent antagonist against all variants of SARS-CoV-2 tested, suggesting it will be active against the emergence of future mutants. Structure-guided engineering of a trimeric ACE2 viral decoy reveals high affinity for spike protein, viral inhibition, and serum stability, offering a universal therapeutic strategy against SARS-CoV-2 variants and SARS-CoV-1 through symmetry-matched avidity. Published in Communications Biology (Oct. 6, 2025): https://doi.org/10.1038/s42003-025-08819-w

|
Scooped by
Juan Lama
September 30, 11:32 AM
|
Creating international viral biosafety guidelines are key to clearing up confusion, regaining trust and ensuring that essential research continues. In an opinion piece published in The New York Times in March, two leading virologists argued that experiments on a coronavirus found in bats and similar to those that cause Middle East respiratory syndrome (MERS) had been conducted without sufficient safety measures. The experiments involved infecting human cells with the live bat virus to see how the virus behaved. Regulatory authorities in many countries where research on potentially dangerous viruses is conducted, including the United States, the United Kingdom, France and Germany, would require such studies to be conducted in biosafety level (BSL) 3 or 4 facilities. (There are four biosafety levels, with BSL-4 being the most stringent.) But for these experiments — which were approved by the equivalent authorities in China — the 25 researchers across 7 institutes and universities in China (including the Guangzhou National Laboratory and the Wuhan Institute of Virology) used BSL-2 procedures. They also used a negative pressure ventilation system designed to prevent microorganisms from spreading outside the laboratory. In our view, this work and the discussion it has provoked highlights a broader and growing problem that the entire virology community needs to address. On the one hand, the threat posed by emerging infectious diseases is growing (see ‘A growing threat’), making investigations of potentially dangerous viruses more important. On the other hand, since the COVID-19 pandemic, trust in virology and science more broadly has declined and work on viruses has become more politicized. To improve trust in science — and to ensure that essential work on viruses can continue — international, standardized and transparent biosafety guidance is urgently needed. Here, we lay out how such guidance might be developed...

|
Scooped by
Juan Lama
September 26, 1:32 PM
|
In a groundbreaking advancement in virology, scientists have unveiled the elusive mechanism by which Epstein–Barr virus (EBV), a pervasive human herpesvirus, infects epithelial cells. This discovery centers on the identification of desmocollin 2 (DSC2) as the principal receptor facilitating EBV entry into epithelial cells—a critical insight that reshapes our understanding of EBV transmission and paves the way for novel therapeutic interventions. EBV, long recognized for infecting B lymphocytes and epithelial cells, has posed an enigma due to the inefficiency of cell-free infection in epithelial tissues despite their susceptibility in vivo. The research unravels this paradox, highlighting the importance of direct cell-to-cell contact and the pivotal role of DSC2 in enabling efficient viral spread. EBV is notorious for its dual tropism, infecting both B cells and the epithelial linings of the oropharynx. While infection of B cells has been extensively studied, epithelial infection mechanisms have remained less clear. Historically, EBV infection of epithelial cells using free viral particles was shown to be inefficient in laboratory settings, yet clinical manifestations suggest otherwise. Prior to this study, some candidates like EphA2 had been proposed as receptors facilitating epithelial infection, but their roles were inconsistent and failed to fully explain infection dynamics. The present study, utilizing an innovative genome-wide CRISPR-Cas9 screen, identified DSC2 as a receptor indispensable for the viral entry process in epithelial cells. The researchers conducted a comprehensive CRISPR screen aimed at pinpointing host factors that facilitate EBV entry into epithelial cells. This unbiased approach allowed them to systematically disable genes and observe the consequences on EBV infection efficiency. Through meticulous analysis, DSC2 emerged as a top candidate, with its knockout resulting in a significant decrease in viral infection rates. Intriguingly, desmocollin 3 (DSC3), a protein closely related to DSC2, was also implicated as a co-factor, not merely a redundant homolog. Together, DSC2 and DSC3 form a critical entry complex, necessary for both cell-free and — importantly — cell-to-cell contact infection modes. Building on these genetic insights, the team then employed loss- and gain-of-function experiments to validate DSC2’s role. Keratinocytes deficient in DSC2 and DSC3 showed a pronounced reduction in infection rates, both when exposed to cell-free viral particles and when co-cultured with EBV-infected B cells. Moreover, overexpressing DSC2 and DSC3 in receptor-negative cells significantly enhanced their susceptibility to infection, providing compelling evidence of their sufficiency and necessity. This dual requirement hints at a sophisticated viral entry mechanism optimized for the unique architecture of epithelial tissues, which are characterized by intricate cell-to-cell contacts. The therapeutic potential of targeting DSC2 was elegantly demonstrated by the application of monoclonal antibodies aimed at this protein. When epithelial cells were treated with antibodies directed at DSC2, EBV infection was markedly inhibited across a range of models, including normal oral keratinocytes, primary oral keratinocytes, and advanced head and neck epithelial organoids. The blockade effect became even more pronounced with a combination of antibodies against both DSC2 and DSC3, which efficiently suppressed the intimate cell-to-cell viral transfer that likely dominates natural infections. This points toward DSC2 as a highly promising target for preventative strategies, including vaccine development and antibody-based therapeutics. Mechanistically, DSC2’s interaction with the viral glycoprotein complex gH/gL was interrogated to unravel the intricacies of EBV fusion and entry. The study found that DSC2 directly binds to gH/gL, facilitating the membrane fusion process that enables viral capsid delivery into the host cytoplasm. This interaction is critical, as it orchestrates the structural rearrangements needed for EBV to breach the epithelial cell membrane. Interestingly, attempts to rescue infection in cells lacking DSC2 and DSC3 by overexpressing EphA2, a previously proposed EBV receptor, failed—highlighting a dependency hierarchy and confirming DSC2/3 as the dominant receptor complex for epithelial infection. The implication of these findings extends beyond basic virology, impacting our understanding of EBV-associated malignancies. EBV’s ability to exploit the DSC2 receptor complex for infection suggests that disruptions or variations in desmosomal components could influence susceptibility to infection and subsequent oncogenic transformation. Since EBV is causally linked to several epithelial malignancies, including nasopharyngeal carcinoma and certain head and neck cancers, targeting the DSC2 interaction axis holds potential not just for infection prophylaxis but also for interrupting oncogenic progression. Furthermore, the discovery refines the model of EBV pathogenesis within the oral cavity and oropharynx. The efficient transmission via direct B cell and epithelial cell contact underscores the significance of tissue architecture and cellular microenvironments in viral persistence and dissemination. EBV’s preference for this contact-mediated route rather than relying solely on cell-free virions explains longstanding clinical observations and reconciles previous inconsistencies in in vitro infection studies. This study thus bridges significant gaps in viral epidemiology and transmission dynamics. The application of advanced organoid culture systems in this research represents a leap forward in modeling EBV infection in near-physiological conditions. Head and neck epithelial organoids, which recapitulate the complex differentiation and stratification of epithelial tissues, allowed for more accurate assessment of viral entry and spread. The successful inhibition of infection in these organoids using DSC2-targeting antibodies strengthens the translational prospects of these findings, indicating that therapeutic strategies developed in vitro may be applicable in vivo. This study also raises fascinating questions about the broader role of desmosomal cadherins in viral infections. Desmocollins like DSC2 and DSC3 are key components of desmosomes, structures critical for cellular adhesion and tissue integrity. Viruses co-opting these proteins for entry point to a possible convergence of cell adhesion pathways and viral invasion mechanisms. This cross-talk may be exploited by other pathogens and represents a fertile ground for future research exploring host-microbe interactions at cellular junctions. Moreover, the elucidation of DSC2 as a primary receptor challenges prior paradigms that focused on other molecules such as integrins and Eph receptors. By highlighting a direct interaction with the viral glycoprotein complex, this study reorients therapeutic design toward desmosomal proteins, which may have been previously underappreciated. This shift in focus may inspire the generation of novel antiviral drugs that interfere specifically with the fusion process facilitated by DSC2-gH/gL binding. Given the ubiquitous prevalence of EBV and its association with a spectrum of diseases ranging from infectious mononucleosis to malignancies, the identification of DSC2 as the principal epithelial entry receptor offers a universal target. This could translate into the development of broadly applicable vaccines or monoclonal antibody therapies that prevent initial infection or limit viral spread, significantly impacting global health. In the future, clinical trials targeting DSC2 may redefine EBV management, shifting from symptomatic treatment to direct infection blockade. In summary, this pioneering work sheds light on the molecular underpinnings of EBV epithelial infection, introducing desmocollin 2 as the linchpin receptor that facilitates viral entry via direct cell-to-cell contact. The reliance on DSC2 and DSC3 for infection, the demonstrable blockade via antibodies, and the failure to rescue infection through alternative receptors compel a reevaluation of EBV biology. These discoveries have far-reaching implications for virology, oncology, and therapeutic development, marking a paradigm shift in the battle against this ubiquitous virus. The comprehensive investigation by Wang et al. not only clarifies the elusive mechanism of EBV epithelial infection but also inspires an array of future research directions. Understanding the structural basis of the DSC2-gH/gL interaction, exploring how desmosomal integrity influences EBV pathogenesis, and translating these findings into clinical applications are poised to transform the landscape of EBV prevention and treatment. This study epitomizes the power of integrative genomic screening and cellular modeling in unraveling complex viral-host interactions. As scientists continue to unravel the complexities of EBV’s interactions with its human host, the identification of desmocollin 2 as a principal entry receptor is a milestone achievement. It underscores the intricate interplay between viral evolution and host cell biology, revealing how viruses have adapted to exploit cellular machinery to ensure survival and propagation. This breakthrough serves as a blueprint for tackling other viral pathogens with similarly enigmatic infection mechanisms, showcasing how cutting-edge technologies can illuminate biological mysteries with profound clinical impact. Research published in Nat. Microbiology (2025): https://doi.org/10.1038/s41564-025-02126-0

|
Scooped by
Juan Lama
September 25, 11:44 AM
|
Huntington’s disease has been successfully treated for the first time using a gene therapy, which may be available in the US as soon as next year. An experimental gene therapy has become the first treatment to successfully slow the progression of Huntington’s disease. While the findings are still preliminary, the approach could be a major breakthrough and may even lead to new therapies for other neurodegenerative conditions, like Parkinson’s and Alzheimer’s. How does the therapy work? The treatment, called AMT-130, targets abnormal proteins in the brain that are responsible for the progression of Huntington’s disease. People with the condition have a genetic mutation that causes the normally-benign huntingtin protein to accumulate in toxic clumps inside brain cells, ultimately killing them. Over time, this leads to memory loss, difficulty walking, slurred speech and other symptoms. The experimental therapy, developed by the Dutch biotechnology company uniQure, stops the production of these mutant proteins. It does so by delivering genetic material to brain cells packaged inside a harmless virus. This material then directs cells to produce a small genetic molecule, called microRNA, which is designed to intercept and disable the instructions for producing the toxic protein. Think of it like a molecular stop signal. The shocking discovery that our gut microbiome drives ageing How and where is the treatment delivered? The treatment targets two brain regions first impacted by Huntington’s disease: the caudate nucleus and the putamen. Both are located deep inside the brain, so doctors use real-time brain scans to guide a thin catheter into them. The entire procedure takes 12 to 18 hours. One injection seems to be enough to permanently lower levels of mutant huntingtin in the brain...

|
Scooped by
Juan Lama
September 22, 11:45 AM
|
In a Q&A with STAT, Nobel winner Drew Weissman addressed concerns raised about Covid shots at a recent meeting of federal vaccine advisers. Drew Weissman Refutes RFK Jr. Adviser's Claims About Covid Shots. On Thursday and Friday, the Centers for Disease Control and Prevention held a meeting of its Advisory Committee on Immunization Practices, a key panel that gives the CDC’s director guidance about what vaccines to recommend to the public. The panel, in the end, postponed its most controversial vote and made recommendations that seemed aimed at fostering doubts about the Covid-19 vaccines while still keeping them widely available. But some presenters and panelists raised concerns that the mRNA Covid vaccines — the ones made by Moderna and Pfizer/BioNTech — may have unconfirmed safety issues. In particular, Retsef Levi, who chairs ACIP’s working group on Covid vaccines, raised concerns that mRNA, the lipid nanoparticles they are encapsulated in, and the spike protein the vaccines produce may all persist and be widely distributed in the body. He also said they may provoke an immune response that is not understood and could even change the way the body reads its own genetic material. Moderna and Pfizer said that the claims were refuted by well-done studies that met global regulatory standards. STAT posed questions about these claims to Drew Weissman, a professor at the University of Pennsylvania and co-recipient of the 2023 Nobel Prize in physiology or medicine for his discoveries that enabled the creation of mRNA-based Covid-19 vaccines. That conversation, edited for length and clarity, follows. At the ACIP meeting, there were a lot of discussions about reports of mRNA being widely distributed in the body or persisting. If you look in the literature you can find papers that say that the earth is flat, you can find papers that say DNA isn’t double stranded. You can find anything. The problem is there’s thousands, tens of thousands, hundreds of thousands, millions of other papers that refute that. What these people do, is that they search, they find one paper or two papers that make an outlandish claim based on bad data that hundreds or thousands or tens of thousands of other papers refute, they don’t mention everything that refutes it. They only mention, oh, look, I found a paper that says spike is around for nine months! It’s just not true. Many good studies have not seen that. The RNA is gone in days. It doesn’t go to the brain. It doesn’t go to the eyes. What those studies did is they put huge doses of RNA into a mouse and used very sensitive assays, and that’s where it went. It goes everywhere. If you put a vaccine equivalent dose, you see it in the muscle, you see it in the draining lymph node, and that’s about it. But how certain are we that mRNA is not continuing to produce spike protein? For instance, the preprint authored by Akiko Iwasaki, which includes well-respected Yale researchers. That’s a paper that found at least residual spike protein. The problem is they used a bad assay to measure spike. It’s an ultra, ultra-sensitive assay, but it makes a lot of errors, and you don’t believe anything unless it’s above a certain level as real. What they reported was in the unknown area where it could just be noise in the assay versus real spike. Many other assays using good experiments, using good assays, don’t find spike protein circulating.
How sure are you? How sure are you that there is no case of mRNA being distributed more widely in the body? I know it’s not distributed widely. I mean, we showed that back in 2017 at vaccine doses. We’ve looked at mice, we’ve looked at macaques, we’ve looked at rabbits, and we’ve done as much as we could on humans at a vaccine dose. You don’t see RNA circulating in the placenta, in the testes, in the heart, in the eye, in the brain, all the places that they list. We and many others have looked and we just don’t see it. And the mRNA could not be persistent? Could one dose of mRNA continue to make spike protein for months in a rare patient? It is absolutely impossible. MRNA is degraded incredibly rapidly. When you modify it, it’s a little slower. It’ll last 24 hours. It never, ever lasts six months. That’s just impossible. But I can make an assay that gives you any answer you want, and that’s what these people have done. I’d like to read specifically from the concerns about mRNA vaccines made by Dr. Retsef Levi, the chair of the working group for ACIP. We covered his concern about “wide biodistribution and prolonged persistence of spike, mRNA and nano-lipid particles.” He also wrote that there are prolonged immune responses that are not understood.
In people who are vaccinated, it was incredibly rare to see that. It was mostly immunized mice. You can always find people who react differently. You can always find people who don’t respond, for instance. Another concern is a “frame shift leading to production of unintended proteins and related immune response,” meaning the vaccine has changed the way that DNA is read to make proteins. That was one paper that claimed that uridine caused a frameshift mutation and induced immune responses. It was never seen in thousands and thousands of other studies. What about the issue of DNA contamination — that DNA impurities are present at too high a level and that the lipoprotein that allows the mRNA to get into cells could lead to them being taken up? Just about every vaccine has DNA contamination. If it is made from eggs, if it is made from living cells, if it is an inactivated virus, if it’s a live virus. They’re all grown in cells and cells have DNA. So when you purify the virus, you get very low levels of DNA contamination. We’ve never seen an adverse event. And these are minute amounts. For mRNA, the DNA strands are a couple of nucleotides long. We’ve never seen them integrate into the chromosomes or cause cancer, it just hasn’t been seen. People give a milligram dose of DNA plasmid as a vaccine, it’s in clinical trials right now. We don’t see any adverse events from that. Is there anything else you’d like to say? What concerned me more from these meetings is they voted against combined childhood vaccines. They voted against making Covid-19 vaccines available to larger populations. They’re going to likely vote against giving hepatitis B vaccines to infants. So if you look back at the data, 250 years ago, 40% of kids never made it to adulthood. Today, it’s 4%. The majority of that decrease is due to vaccines. Vaccines have saved more lives than any other type of medical intervention, including antibiotics, including everything. Vaccines have saved an enormous number of lives. No vaccine was tested more extensively than the RNA vaccines, and no vaccine was given to more people than the mRNA vaccines, and they were found to be incredibly safe, safer than any other vaccine platform and effective. They saved 20 million lives, and they stopped a pandemic that was shutting down the world.
|

|
Scooped by
Juan Lama
October 31, 11:53 AM
|
Children may be more likely to be diagnosed with autism and other neurodevelopment disorders if their mother had a Covid-19 infection while pregnant, according to a new study. Researchers from Massachusetts General Hospital analyzed more than 18,000 births that occurred in the Mass General Brigham health system between March 2020 and May 2021, assessing records for laboratory-confirmed Covid-19 tests among the mothers and for neurodevelopment diagnoses among their children through age 3. They found that children born to mothers who had Covid-19 during pregnancy were significantly more likely to be diagnosed with a neurodevelopment disorder than those born to mothers who did not have an infection while pregnant: more than 16% versus less than 10%, or a 1.3 times higher risk after adjusting for other risk factors. Overall, differences in risks were more pronounced among boys and in cases where the mother had a Covid-19 infection during the third trimester. Previous studies have suggested that male fetal brains are more susceptible to maternal immune responses, according to the authors of the new study, and the third trimester is a “critical window for brain development.” The most common diagnoses included disorders in speech and motor function development and autism. About 2.7% of children born to mothers who had Covid-19 while pregnant were diagnosed with autism, compared with about 1.1% of others, according to the study, published Thursday in the journal Obstetrics and Gynecology. The new findings are “particularly notable in light of their biological plausibility,” the researchers wrote. They build on previous research that identified potential pathways for a maternal Covid-19 infection to affect the developing fetal brain even without direct transmission. “Parental awareness of the potential for adverse child neurodevelopmental outcomes after COVID-19 in pregnancy is key. By understanding the risks, parents can appropriately advocate for their children to have proper evaluation and support,” Dr. Lydia Shook, maternal-fetal medicine specialist at Massachusetts General Hospital and lead author of the study, said in a news release. About 1 in 31 children in the US was diagnosed with autism by age 8 in 2022, according to a report published by the US Centers for Disease Control and Prevention that was published in April. The increase — up from 1 in 36 children in 2020 — continues a long-term trend that experts have largely attributed to better understanding of and screening for the condition. Earlier this year, the US Department of Health and Human Services launched a “massive testing and research effort” to determine “what has caused the autism epidemic.” In a news conference in September on the “cause of autism,” President Donald Trump — flanked by HHS Secretary Robert F. Kennedy Jr. and other federal health leaders – said that use of Tylenol during pregnancy can be associated with a “very increased risk of autism,” despite decades of evidence that it is safe. Kennedy also has a history of comments linking autism and vaccines, despite strong evidence that the two are not connected. The timeframe of the new study – early in the pandemic, before vaccines were widely available – meant that the researchers were able to “isolate the association between SARS-CoV-2 infection and offspring neurodevelopment in an unvaccinated population.” About 93% of the mothers included in the assessment had not received any doses of Covid-19 vaccine. Strong infection-control policies at that time also helped reduce the potential for unreported or undetected Covid-19 cases, the researchers said. “These findings highlight that COVID-19, like many other infections in pregnancy, may pose risks not only to the mother, but to fetal brain development,” Dr. Andrea Edlow, a maternal-fetal medicine specialist at Mass General Brigham and senior author of the new study, said in a news release. “They also support the importance of trying to prevent COVID-19 infection in pregnancy and are particularly relevant when public trust in vaccines – including the COVID-19 vaccine – is being eroded.” Study published Oct. 30, 2025:

|
Scooped by
Juan Lama
October 29, 10:33 AM
|
Research Highlights: - A review of 155 scientific studies found influenza and COVID infections raised the risk of heart attack or stroke as much as three-to five-fold in the weeks following the initial infection.
- Viruses that linger in the body, such as HIV, hepatitis C and varicella zoster virus (the virus that causes shingles), can lead to long-term elevations in the risk of cardiovascular events.
- The study researchers say preventive measures, including vaccination, may play an important role in reducing the risk of heart attacks and strokes, especially in people who already have heart disease or heart disease risk factors.
In the weeks following a bout of influenza or COVID, the risk of heart attack or stroke may rise dramatically, and chronic infections such as HIV may increase the long-term risk of serious cardiovascular disease events, according to new, independent research published today in the Journal of the American Heart Association, an open access, peer-reviewed journal of the American Heart Association. “It is well recognized that human papillomavirus (HPV), hepatitis B virus and other viruses can cause cancer; however, the link between viral infections and other non-communicable diseases, such as cardiovascular disease, is less well understood,” said Kosuke Kawai, Sc.D., lead author of the study and adjunct associate professor in the division of general internal medicine and health services research at the David Geffen School of Medicine at the University of California, Los Angeles. “Our study found acute and chronic viral infections are linked to both short- and long-term risks of cardiovascular disease, including strokes and heart attacks.” The researchers set out to systematically review all published studies that investigated the association between any viral infection and the risk of stroke and heart attack, initially screening more than 52,000 publications and identifying 155 as appropriately designed and of high quality allowing for meta-analysis of the combined data. In studies that compared people’s cardiovascular risks in the weeks following documented respiratory infection vs. the same people’s risk when they did not have the infection, researchers found: - People are 4 times as likely to have a heart attack and 5 times more likely to have a stroke in the month after laboratory-confirmed influenza.
- People are 3 times more likely to have a heart attack and 3 times as likely to have a stroke in the 14 weeks following COVID infection, with the risk remaining elevated for a year.
The immune system’s natural response to viral infections includes the release of molecules that trigger and sustain inflammation and promote the tendency of blood to clot, both of which may last long after the initial infection has been resolved. Both inflammation and blood clotting can reduce the ability of the heart to function properly and may help explain the increased heart attack and stroke risk. Inflammation plays a key role in the development and progression of cardiovascular disease (CVD). It contributes to the formation and rupture of plaques in arteries, which can lead to heart attacks and strokes. Some elevated inflammatory markers are linked to worse outcomes and higher risk of future events; thus, managing inflammation is becoming an important part of preventing and treating CVD. In studies comparing long-term risk (average of more than 5 years) of cardiovascular events in people with certain chronic viral infections vs. similar people without the infection, the researchers found: - A 60% higher risk of heart attack and 45% higher risk of stroke in people with HIV infection.
- A 27% higher risk of heart attack and 23% higher risk of stroke in people with hepatitis C infection.
- A 12% higher risk of heart attack and 18% higher risk of stroke in people had shingles.
“The elevated risks for cardiovascular disease risks are lower for HIV, hepatitis C and herpes zoster than the heightened short-term risk following influenza and COVID. However, the risks associated with those three viruses are still clinically relevant, especially because they persist for a long period of time. Moreover, shingles affects about one in three people in their lifetime,” Kawai said. “Therefore, the elevated risk associated with that virus translates into a large number of excess cases of cardiovascular disease at the population level.” The findings also suggest that increased vaccination rates for influenza, COVID and shingles have the potential to reduce the overall rate of heart attacks and strokes. As an example, the researchers cite a 2022 review of available science that found a 34% lower risk of major cardiovascular events among participants receiving a flu shot in randomized clinical trials vs. participants in the same trials who were randomly selected to receive a placebo instead. “Preventive measures against viral infections, including vaccination, may play an important role in decreasing the risk of cardiovascular disease. Prevention is especially important for adults who already have cardiovascular disease or cardiovascular disease risk factors,” Kawai said. According to the American Heart Association, people may be at greater risk for cardiovascular disease because of viruses such as influenza, COVID, RSV and shingles. Additionally, because people with cardiovascular disease may face more severe complications from these viruses, the Association recommends those individuals consult with a health care professional to discuss which vaccines are right for them, as vaccination offers critical protection to people already at increased risk. Although a connection has been suggested in previous studies, researchers note there is currently limited evidence and more studies are needed to understand the possible links between heart disease risk and several other viruses, including cytomegalovirus (virus that can cause birth defects), herpes simplex 1 (virus that causes cold sores), dengue (mosquito-spread virus that can cause dengue fever) and human papilloma virus (can cause cervical and other cancers later in life).
The current analysis has some limitations as it was based on observational studies rather than randomized controlled trials; however, many of the studies accounted adequately for potential confounding factors. Because most studies examined infection with a single virus, it is unclear how infection with multiple viruses or bacteria may have affected the results. The analysis focused on viral infections that impact the general public and did not identify high-risk groups (such as transplant recipients) that may be disproportionately affected. Study details, background and design: - Investigators searched multiple medical databases from inception through July 2024 for studies examining the association of viral infections and cardiovascular diseases, then screened 52,336 possibly relevant publications and selected 155 studies as appropriate for analysis.
- Studies were published between 1997 and 2024 and most were conducted in North America (67), Europe (46) and East Asia (32).
- 137 studies evaluated one viral infection and 18 studies evaluated 2 or more.
- For each virus under consideration, researchers performed a meta-analysis of studies employing the same study design.
Co-authors, disclosures and funding sources are listed in the manuscript. Studies published in the American Heart Association’s scientific journals are peer-reviewed. The statements and conclusions in each manuscript are solely those of the study authors and do not necessarily reflect the Association’s policy or position. The Association makes no representation or guarantee as to their accuracy or reliability. The Association receives more than 85% of its revenue from sources other than corporations. These sources include contributions from individuals, foundations and estates, as well as investment earnings and revenue from the sale of our educational materials. Corporations (including pharmaceutical, device manufacturers and other companies) also make donations to the Association. The Association has strict policies to prevent any donations from influencing its science content and policy positions. Overall financial information is available here.

|
Scooped by
Juan Lama
October 25, 11:12 AM
|
Mammalian cells employ a wide array of antiviral defense mechanisms to restrict viral replication at virtually all steps of the viral life cycle. Notably, the interferon (IFN) response has been shown to play a central role in restricting the replication of disparate viral pathogens in mammals. Consequently, since its discovery in 1957, the IFN response has dominated antiviral immunity research, leaving IFN-independent pathways relatively understudied. Exploring these alternative host defenses is crucial for understanding the complete arsenal that mammalian hosts deploy to combat viral disease, as IFN responses undoubtedly work in concert with other antiviral defenses to achieve virus restriction. Here, we discuss selected examples of antiviral factors and pathways in mammals that are not classically associated with the IFN response. These defenses range from constitutively expressed host restriction factors that directly inhibit specific steps of the viral life cycle to signaling pathways that invoke IFN-independent antiviral gene expression programs to cell death mechanisms that sacrifice the infected cell to prevent viral spread. Ultimately, our goal is to highlight the diversity of IFN-independent antiviral defenses that mammalian hosts utilize to block viral infection. Published in J. Virology (Oct. 2025):

|
Scooped by
Juan Lama
October 23, 12:33 PM
|
mRNA vaccines seem to boost the effectiveness of an immune therapy for skin and lung cancer ― in an unexpected way. A vaccine that helps to fight cancer might already exist. People being treated for certain deadly cancers lived longer if they had received an mRNA-based vaccine against COVID-19 than if they hadn’t, finds an analysis of medical records. Follow-up experiments in mice show that the vaccines have this apparent life-extending effect not because they protect against COVID-19 but because they rev up the body’s immune system1. That response increases the effectiveness of therapies called checkpoint inhibitors, the animal data suggest. “The COVID-19 mRNA vaccine acts like a siren and activates the immune system throughout the entire body”, including inside the tumour, where it “starts programming a response to kill the cancer”, says Adam Grippin, a radiation oncologist at MD Anderson Cancer Center in Houston, Texas, an co-author of the report published today in Nature. “We were amazed at the results in our patients.” The findings, which Grippin and his colleagues hope to validate in a clinical trial, suggest further hidden capabilities of mRNA vaccines, even as the administration of US President Donald Trump has slashed about US$500 million in funding for research investigating the technology. The US Department of Health and Human Services, which cancelled the funding for mRNA research, did not respond to a request for comment. Working in tandem Checkpoint inhibitors unleash the immune system to attack cancer cells. They have transformed the treatment of many cancers, but they fail in more than half of the people who receive them: some recipients’ immune systems remain too sluggish to attack cancer cells. To address this gap, researchers have been developing personalized ‘cancer vaccines’. These would be used in tandem with checkpoint inhibitors to help an individual’s immune system to target the unique mutations found in their cancer cells. Although early results are promising, these treatments are still experimental and, once available, will probably be very expensive and difficult to access. Grippin and his colleagues wondered whether the general immune boost that mRNA vaccines create could be enough to wake up the immune system. They found support for this theory in mice2, leading them to investigate whether the effect would carry over into people. The researchers analysed the medical records of more than 1,000 people with lung cancer or melanoma. They found that, in people with a certain type of lung cancer, receiving an mRNA COVID-19 vaccine was linked to a near doubling in survival time, from 21 months to 37 months. Unvaccinated people with metastatic melanoma survived an average of 27 months; by the time data collection ended, vaccinated people had survived so long that the researchers couldn’t calculate an average survival time. People whose tumours had traits hinting that they were unlikely to respond to checkpoint inhibitors saw the biggest survival boost after vaccination. This finding is “quite impressive”, says Benoit Van den Eynde, a tumour immunologist at the University of Oxford, UK. “I did not expect the effect to be that significant, and the data are very strong.” Window of opportunity Timing matters: those who had received the jab within 100 days of starting treatment were more likely to benefit than were those who received it outside that window. Grippin has collected data, which are yet to be published, suggesting that receiving the vaccine within a 30-day window before or after treatment could elicit an even stronger boost, he says. This survival benefit was not seen with vaccines that do not use mRNA technology, such as those for influenza and pneumonia, or in people who received a different type of cancer therapy. The follow-up experiments in mice hinted at an explanation for this increase in survival. mRNA vaccines comprise mRNA encased in fatty nanoparticles, which deliver their payload directly into cells. The combination of the fatty particles and the insertion into cells leads to potent activation of the immune system. Vaccination leads to the activation of a cascade of immune cells, which trains the body’s ‘killer’ cells to hunt for tumour cells. These killer cells are then aided by the checkpoint-inhibitor drugs, the researchers found. Maligned technology These data suggest that a measure that is both widely available — billions of doses of mRNA COVID-19 vaccine have been distributed globally — and also low-cost could help to boost survival in people with a wide array of cancers, Van den Eynde says. That doesn’t mean researchers should throw away years of investment and research into personalized cancer vaccines, Grippin says. If this approach is shown to be effective in clinical trials, Grippin says that two types of vaccine could be used simultaneously — one to stimulate a general immune response and another to train the immune system to fight cancer cells specifically. But more data would require research in a field that has been defunded and criticized by Trump-administration officials. “The current climate impacts patients because even the word, ‘mRNA’, has stigma these days,” says study co-author Steven Lin, an oncologist at MD Anderson. “We’re walking on eggshells because there’s so much negative publicity about mRNA.”

|
Scooped by
Juan Lama
October 20, 11:41 AM
|
Mexico has reported a new human case of H5 avian influenza in a 23-year-old woman in Mexico City, according to health officials. The patient has since been released from the hospital. The woman, who had no recent history of travel, began developing symptoms on September 14, according to the Pan American Health Organization (PAHO). She was later admitted to a hospital in the country’s capital. Her illness began with respiratory symptoms, including a runny nose and cough, which progressed to fever, painful swallowing, and later hemoptysis (coughing up blood) and chest pain. A sample collected on September 29 tested positive for unsubtypeable influenza A, and the presence of influenza A(H5) was confirmed by real-time RT-PCR the following day, PAHO said. She was treated with oseltamivir and discharged on October 11. Health authorities said a dog lived at the woman’s residence, and several birds were present in the building’s courtyard, including a poultry bird and two pigeons. Bird droppings were also found in multiple areas, including a poorly sealed cistern that supplied water to all apartments in the building. Samples collected from the animals tested positive for influenza A(H5), while environmental samples are still being analyzed. Tests from 41 identified contacts of the patient were all negative for the virus, according to officials. It remains unclear which H5 subtype caused the infection. Mexico’s first reported human case of avian influenza occurred in 2024 and involved the H5N2 strain, which led to the death of a 59-year-old man in the neighboring State of Mexico. Earlier this year, the country reported its first H5N1 case, a 3-year-old girl from a rural area in northern Mexico who died after severe complications. Genetic analysis identified the strain as Genotype D1.1. This genotype has also been detected in at least five human cases across North America, including the fatal case of a person in Louisiana in 2024, the first confirmed H5N1 death in the United States. It also caused severe illness in a teenager in Canada and in an adult in Wyoming in February. The spread of H5N1 clade 2.3.4.4b and its multiple genotypes has raised concern among global health experts due to its wide geographic distribution and ability to infect both birds and mammals. Since 2022, at least 92 human infections with this clade have been reported worldwide, most linked to contact with infected poultry or dairy cattle.

|
Scooped by
Juan Lama
October 17, 1:48 PM
|
In March 2024, a highly pathogenic avian influenza H5N1 (HPAI) clade 2.3.4.4b virus was identified in US dairy cows, with spillover to cats, poultry, and humans. Up to 30% of commercial pasteurized milk tested contained viral genome copies. The impact of residual viral remnants on host immunity is unknown. Orally ingested proteins can stimulate gut-associated lymphoid tissues, potentially inducing tolerance and altering responses to later infection. We found that milk pasteurization fully inactivated pandemic H1N1 and bovine H5N1 influenza viruses yet preserved hemagglutinin (HA) protein integrity. In mice, repeated oral exposure to inactivated virus did not alter mortality after H5N1 virus challenge. Preliminary data showed that naïve mice exposed to improperly pasteurized milk containing live H5N1 virus developed lethal infection, whereas prior H1N1 infection conferred protection. Mice with preexisting H1N1 immunity remained protected when challenged with bovine H5N1 virus after exposure to H5N1 pasteurized in milk. These findings suggest that pasteurized milk containing inactivated H5N1 virus poses minimal health risks. Published in Science Advances: https://doi.org/10.1126/sciadv.aeb3906

|
Scooped by
Juan Lama
October 12, 12:48 PM
|
Friday’s layoffs swept up scientists involved in responding to disease outbreaks and running an influential journal. Officials said the mistaken dismissals were being rescinded. The Trump administration on Saturday raced to rescind layoffs of hundreds of scientists at the Centers for Disease Control and Prevention who were mistakenly fired on Friday night in what appeared to be a substantial procedural lapse. Among those wrongly dismissed were the top two leaders of the federal measles response team, those working to contain Ebola in the Democratic Republic of Congo, members of the Epidemic Intelligence Service, and the team that assembles the C.D.C.’s vaunted scientific journal, The Morbidity and Mortality Weekly Report. After The New York Times reported the dismissals, two federal health officials said on Saturday that many of those workers were being brought back. The officials spoke anonymously in order to disclose internal discussions. The mistakes rocked an agency already in tumult, and which has been a particular target of Health Secretary Robert F. Kennedy Jr. The C.D.C. lost about a third of its staff in April; many were rehired weeks later. In August, a gunman emptied more than 500 rounds of ammunition at the agency’s headquarters in Atlanta. Later that month, Mr. Kennedy orchestrated the ouster of the agency’s director, Susan Monarez, and precipitated a series of high-profile resignations. Among the workers whose firings were revoked were members of the elite corps of “disease detectives” who are typically deployed to the sites of outbreaks. The team that puts together the M.M.W.R., which communicates the agency’s recommendations and research, has also been brought back. The employees “were sent incorrect notifications, which was fixed last night and this morning with a technical correction,” a senior administration official said. “Any correction has already been remedied.” In order to ensure that teams confronting disease outbreaks include scientists with varied expertise, they comprise staff from various parts of the agency. The two top leaders of the measles response, for example, are officially employees of the office of the director at the Global Health Center, and the office of the director at the National Center for Immunization and Respiratory Diseases. When outbreaks die down, team scientists return to their regular positions. The leaders of the measles team were let go when the administration eliminated those two offices. But just as entire units must be cut in such a layoff, entire units must also be restored. Athalia Christie, who was “incident commander” of the measles response, had nearly 30 years of experience managing outbreaks, including Ebola, Marburg and mpox, previously called monkeypox. The White House often reached out to her for help with outbreaks. “Athalia is very well liked by the administration,” said Dr. Demetre Daskalakis, who led the respiratory disease center before he resigned in August. He had brought in Dr. Christie to lead the measles response. Another senior infectious disease expert, Maureen Bartee, was working at the Department of State. But both their jobs fell under the director’s office of the C.D.C.’s Global Health Center, which was eliminated in the layoffs. By Saturday night, employees of both offices, including Dr. Bartee and Dr. Christie, had received notices of their rehiring. They and others received a two-paragraph email saying that the notice they had received “on or about” Oct. 10 had been revoked. “You will not be affected by the upcoming RIF,” the email said. The confusion over how the disease teams are organized “demonstrates their lack of understanding that this thing is an interconnected organism,” Dr. Daskalakis said, referring to the C.D.C. “I’m happy people are back, but this damage is not easy to repair both for current staff and for people who will lead public health in the future,” he added. The agency’s entire Washington office, which was laid off on Friday, will not be rehired. Nor will employees of the office of the director of the center for injury prevention, or those at the division of violence prevention policy. “This is going to be devastating to Americans and to the global community,” said Dr. Debra Houry, who served as the agency’s chief medical officer before she resigned in August in protest against the administration’s policies. “They are dismantling public health,” she added.

|
Scooped by
Juan Lama
October 8, 12:07 PM
|
Background Clesrovimab is a long-acting investigational monoclonal antibody against site IV of the respiratory syncytial virus (RSV) fusion protein. Data regarding the safety and efficacy of clesrovimab in healthy infants are needed. Methods We randomly assigned healthy preterm and full-term infants entering their first RSV season in a 2:1 ratio to receive one intramuscular 105-mg dose of clesrovimab or placebo. The primary efficacy end point was RSV-associated medically attended lower respiratory infection (including at least one indicator of lower respiratory infection or disease severity) through 150 days after injection. A key secondary efficacy end point was RSV-associated hospitalization during the same period. Results A total of 3614 infants received an injection: 2412 infants received clesrovimab, and 1202 infants received placebo. Through day 150 after injection, RSV-associated medically attended lower respiratory infection occurred in 60 of 2398 infants in the clesrovimab group (incidence rate over 5-month period, 2.6%) and in 74 of 1201 infants in the placebo group (incidence rate over 5-month period, 6.5%), for an efficacy of 60.4% (95% confidence interval [CI], 44.1 to 71.9; P<0.001). RSV-associated hospitalization within 150 days was reported in 9 of 2398 infants in the clesrovimab group and in 28 of 1201 infants in the placebo group, for an efficacy of 84.2% (95% CI, 66.6 to 92.6; P<0.001). Serious adverse events were reported in 278 of 2409 infants (11.5%) in the clesrovimab group and 149 of 1202 infants (12.4%) in the placebo group. Conclusions In healthy preterm and full-term infants, a single dose of clesrovimab reduced the incidence of RSV-associated medically attended lower respiratory infection and RSV-associated hospitalization, with a safety profile similar to that of placebo. Published (Sept. 17, 2025):

|
Scooped by
Juan Lama
October 5, 11:51 AM
|
After reacquiring the rights to its non-vaccine flu preventative last year, Cidara Therapeutics has secured federal support to develop and produce the candidate, dubbed CD388, in the U.S. | The initial part of the up-to-$339 million funding will largely be used to establish U.S. manufacturing for the non-vaccine flu preventive candidate, with additional options tied to further clinical studies, Cidara said. The remaining $281 million in options could be used to support additional studies of CD388 in specific populations, which Cidara said it would position as “a complement to the company’s plans” for an approval application to the FDA. “Clinical and non-clinical data generated to date suggest that CD388 has the potential to be an effective non-vaccine preventative for both pandemic and seasonal influenza,” Jeffrey Stein, Ph.D., Cidara’s chief executive, said in a statement. The BARDA tie-up will “both expand our commercial supply capacity, as well as ensure U.S. supply of CD388 in the event of an influenza pandemic,” he added. CD388 is being developed as a non-vaccine alternative for flu prevention. The prophylactic is a member of the drug-Fc conjugate class, comprised of multiple copies of a potent small molecule neuraminidase inhibitor conjugated to a bespoke Fc fragment of a human antibody, according to Cidara. The company stressed in its release that drug-Fc conjugates are not vaccines or monoclonal antibodies. Rather, they are low molecular weight biologics meant to function as “long-acting small molecule inhibitors,” Cidara said.Cidara is developing CD388 to guard against all known strains of seasonal and pandemic influenza, and, because the candidate is not a vaccine, it’s expected to work in patients regardless of their immune status. Cidara regained control of CD388 from Janssen last April following the Johnson & Johnson unit’s wind-down of all infectious disease and vaccine R&D. At the time of the strategy shift, Janssen had indicated that it planned to transfer rights to the drug to another entity, according to Cidara. To recover its asset, Cidara handed Janssen $85 million upfront in a deal that also includes the potential for development, regulatory and commercial milestone payments. The loss of a Big Pharma partner left Cidara with a funding gap, although the BARDA deal, a private stock placement led by RA Capital Management last year and other recent funding moves are likely helping bridge the gap for the San Diego company. Cidara has announced a slew of CD388 updates over the past few months, starting with a phase 2b data readout on the asset in June. In the study, dubbed Navigate, the highest 450-mg dose of CD388 conferred 76% protection against seasonal influenza in unvaccinated adults versus placebo, while lower doses of 300 mg and 150 mg conferred 61% and 58% protection, respectively.In late September, the company noted that it was moving forward with an “expanded and accelerated development plan” for the asset in which it will seek FDA approval using a single phase 3 study. The company said it aims to enroll 6,000 total subjects in the trial, which it had originally aimed to initiate next spring. Patients in the study will receive either the 450-mg dose of CD388 or placebo, Cidara said last month. The company wasted no time getting started, announcing that it had dosed the first participants in the phase 3 program just one day after affirming its late-stage development strategy.

|
Scooped by
Juan Lama
September 30, 11:10 AM
|
With some analyses generating signs of efficacy, the biotech made the case that the data support further development of the direct-acting antiviral. A phase 2b trial of Enanta Pharmaceuticals’ respiratory syncytial virus (RSV) drug candidate has missed its primary endpoint. But, with other analyses generating signs of efficacy, the biotech made the case that the data support further development of the oral, direct-acting antiviral zelicapavir. Investigators randomized 186 patients to receive zelicapavir or placebo once daily for five days. Enanta later narrowed its efficacy population to include just the 175 patients who tested positive for RSV at a central laboratory. After the treatment period, the biotech tracked participants for a further 28 days to assess the effect of zelicapavir. The time to resolution of RSV lower respiratory tract disease (LRTD) symptoms was statistically no better on zelicapavir than on placebo, causing the trial to miss its primary endpoint. Enanta reported a 0.5-day improvement over placebo in time to the complete resolution of the four LRTD symptoms in the efficacy population. Other analyses linked zelicapavir to bigger improvements in time to symptom resolution. In the efficacy population, all 13 RSV symptoms cleared up 2.2 days faster in the treatment cohort. When looking at all 29 parameters in a patient-reported respiratory infection tool, Enanta found symptoms resolved 3.6 days faster on zelicapavir. The biotech also shared analyses of how zelicapavir compared to placebo in patients who had congestive heart failure, chronic obstructive pulmonary disease or who were aged 75 years or older. In total, 81% of participants met one of the criteria for inclusion in the subgroup analyses. Limiting assessments to those patients, Enanta linked zelicapavir to a three-day improvement in the time to resolution of LRTD symptoms. Time to resolution of the 13 RSV symptoms was 6.7 days shorter on the study drug in the subpopulation. The difference when looking at all 29 parameters was 7.2 days. Enanta shared data on a secondary endpoint that used another scale and on the rates of hospitalization—1.7% for zelicapavir versus 5% for placebo—to further support its argument that the results are positive. That conclusion informed comments by Scott Rottinghaus, M.D., chief medical officer of Enanta, about the next steps. “We believe the totality of these data provides strong rationale for further clinical advancement of zelicapavir,” Rottinghaus said in a statement. “Importantly, we identified multiple potential registrational endpoints for a phase 3 trial.” In a note to clients, Evercore analyst Jonathan Miller lauded the drug's “robust benefit” and stated that “no one in [the] RSV space has ever shown benefit like this with an antiviral before.” Shares in Enanta surged by about 40% to $11.03 by early afternoon on Monday from a closing price of $7.90 on Friday. Enanta's Press Release (Sept. 29, 2025): https://ir.enanta.com/news-releases/news-release-details/enanta-pharmaceuticals-reports-positive-topline-results-its

|
Scooped by
Juan Lama
September 26, 12:59 PM
|
Background Oncogenic viruses cause high-risk cancers in humans and are responsible for nearly 20% of all cancer cases worldwide. Currently, very limited data exists in the realm of wastewater-based viral epidemiology (WBE) of cancer-causing viruses, with existing studies using targeted approaches (i.e PCR-based approaches) which lack scalability. Our study aims to carry out WBE with hybrid-capture probes to detect and track multiple oncogenic viruses simultaneously in wastewater across Texas, USA, overcoming the drawbacks associated with targeted approaches. Methods Here, we used a hybrid-capture approach to detect, filter and sequence oncogenic virus signals from wastewater samples collected over a duration of three years, from May 2022 to May 2025. Once viral reads were sequenced, we utilized established computational tools to characterize reads into their respective virus of origin. Next, viral abundances of each characterized oncogenic virus were tracked over time and read coverage across their genomes was measured using read mapping techniques. Findings We detected six known oncogenic viruses, along with three suspected oncogenic viruses across all sampling locations within Texas. Over three years, viral abundance gradually increased, with distinct peaks and dips over the summer and winter months. The prevalence of high-risk viruses such as HPV and EBV rose sharply, with increases in abundance observed post-2024. We also obtained nearly 100% genome coverage with viral reads captured using a hybrid-capture technique for almost all oncogenic viruses and their types. Interpretations Our study shows that a hybrid-capture method can efficiently overcome the challenges faced with using targeted approaches for WBE. Using this method, we get broader read coverage, coupled with concurrent and consistent real-time tracking dynamics of multiple oncogenic viruses. Our findings also emphasize the persistent circulation and rising prevalence of high-risk cancer-causing viruses, underscoring the need for sustained public health interventions to protect communities and assess viral prevalence in high-risk populations. Preprint in medRxiv (Sept. 18, 2025): https://doi.org/10.1101/2025.09.17.25335998

|
Scooped by
Juan Lama
September 24, 11:45 AM
|
A drug that provides near-perfect protection against H.I.V. with shots just twice a year will be made available at $40 per patient annually in low- and middle-income countries, offering new hope for ending the H.I.V. epidemic. Although current treatments can suppress H.I.V., there is no real cure, making affordable prevention crucial for turning back the virus. About 1.3 million people worldwide become infected with H.I.V. every year. The drug, lenacapavir, is given as two shots every six months. Through two deals announced on Wednesday by philanthropic organizations, the shots would cost the same as daily oral pills to prevent H.I.V., making lenacapavir a realistic choice in countries with constrained resources....

|
Scooped by
Juan Lama
September 19, 12:51 PM
|
Purpose Early detection of HPV-associated oropharyngeal cancer (HPV+OPSCC), the most common HPV cancer in the United States, could reduce disease-related morbidity and mortality, yet currently, there are no early detection tests. Circulating tumor HPV DNA (ctHPVDNA) is a sensitive and specific biomarker for HPV+OPSCC at diagnosis. It is unknown if ctHPVDNA is detectable prior to diagnosis, and thus it’s potential as an early detection test. Methods Plasma samples from the MassGeneralBrigham biobank collected 1.3-10.8 years prior to diagnosis from HPV+OPSCC patients (n = 28) and age- and sex-matched controls (n = 28) were blinded and run on a newly developed and validated multi-feature HPV whole genome sequencing liquid biopsy assay and a validated HPV antibody assay. Results ctHPVDNA results were positive in 22/28 pre-diagnostic samples from HPV+OPSCC cases (sensitivity 79%) with a maximum lead time of 7.8 years. ctHPVDNA results were negative in all controls (0/28 controls, 100% specificity). Diagnostic accuracy was highest within four years of cancer diagnosis and was higher than HPV Ab detection within the same time frame (p-value 0.004). Application of a machine learning model trained and tested on an independent cohort of 306 cases and controls increased the sensitivity of detection to 27/28 cases (overall sensitivity 96%) and the maximum lead time to 10.3 years. Conclusions Circulating tumor HPV DNA can be detected in the blood years prior to diagnosis with HPV+OPSCC, with high specificity, in a case-control cohort of 56 participants. ctHPVDNA detection alone, or in combination with previously identified serological biomarkers may be a feasible approach to early detection of HPV+OPSCC. Published Sept. (10, 2025): https://doi.org/10.1093/jnci/djaf249
|





 Your new post is loading...
Your new post is loading...