Your new post is loading...
 Your new post is loading...

|
Suggested by
LIGHTING
July 12, 2018 6:49 AM
|
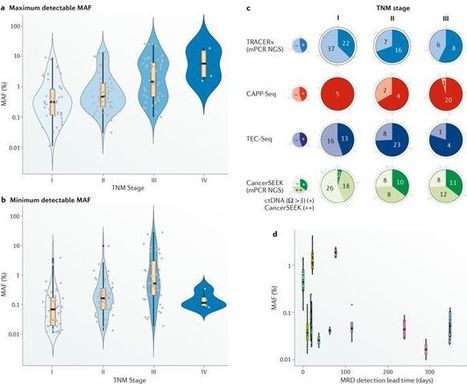
Liquid biopsy approaches hold great promise in early cancer diagnosis or minimal residual disease monitoring for cancer recurrence. Herein, the authors evaluate contemporary next-generation sequencing approaches to circulating tumour DNA detection in these contexts, with a focus on studies in patients with non-small-cell lung cancer. They discuss the feasibility of introducing these strategies into the clinic, highlighting the technical and analytical challenges, as well as possible solutions.

|
Scooped by
Gilbert C FAURE
March 20, 2018 3:26 AM
|
On Friday March 16, 2018, the Centers for Medicare & Medicaid Services (CMS) took action to advance innovative personalized medicine for Medicare patients with cancer. CMS announced it has finalized a National Coverage Determination that covers diagnostic laboratory tests using Next Generation Sequencing (NGS) for patients with advanced cancer. This decision is likely to accelerate …

|
Scooped by
Gilbert C FAURE
October 1, 2017 2:01 PM
|
Picking out circulating tumor cells (CTCs) from whole blood, known as liquid biopsy, should soon be a regular way to screen for cancer and to monitor patie

|
Scooped by
Gilbert C FAURE
September 16, 2017 12:57 PM
|
Liquid biopsy allows cancer investigation without applying invasive procedures to patients

|
Scooped by
Gilbert C FAURE
July 15, 2017 4:08 PM
|

|
Scooped by
Gilbert C FAURE
April 28, 2017 8:15 AM
|
Epic Sciences is one of the coolest kids on the biopharma block, with a new $40 million Series D financing round and a whopping 42 industry partnerships built around its novel liquid biopsy platform. For a team of just 70, the San Diego startup is doing pretty well.

|
Scooped by
Gilbert C FAURE
March 4, 2017 2:49 AM
|
Circulating tumour cells (CTCs) have the potential to act as a source of tumour tissue for the measurement of pharmacodynamic biomarkers in early phase clinical trials.

|
Scooped by
Gilbert C FAURE
December 19, 2016 4:59 AM
|
A chip developed by mechanical engineers at Worcester Polytechnic Institute (WPI) can trap and identify metastatic cancer cells in a small amount of blood drawn from a cancer patient. The breakthrough technology uses a simpl

|
Scooped by
Gilbert C FAURE
October 22, 2016 4:36 AM
|
Detection of circulating tumor cells (CTCs) has become widely used as a liquid biopsy for many patients. In pancreatic cancer patients, there have been a number of published reports on the efficacy of CTCs in the diagnosis and prognosis of .
If immunotherapy has the cancer community riveted on the treatment side, liquid biopsies are equally exciting on the diagnostics end. And IBM is bringing its
Via Krishan Maggon
Nature Medicine | doi:10.1038/nm.4074
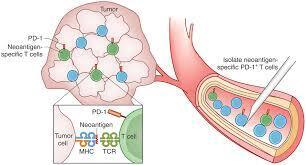
Human T cells that target tumor-specific mutations are attractive for cancer immunotherapy, but obtaining these T cells is challenging. A new study shows that tumor mutation–specific T cells can be isolated from the peripheral blood of patients with melanoma.
Via Krishan Maggon
On June 1, the U.S. Food and Drug Administration (FDA) approved a liquid biopsy test, a companion diagnostic test called cobas EGFR Mutation Test v2. The test uses plasma samples ...
Via Krishan Maggon

|
Scooped by
Gilbert C FAURE
April 9, 2016 9:34 AM
|
People with cancer have tumor DNA in their blood. A new way to quiet background 'noise' in the blood sample allows researchers to sequence minute quantities of these molecules to improve diagnosis and treatment.
|

|
Scooped by
Gilbert C FAURE
April 14, 2018 4:11 AM
|

|
Scooped by
Gilbert C FAURE
March 14, 2018 12:59 PM
|
Scientists reported the development of a robust procedure for whole-genome copy number profiling of circulating tumor cells (CTCs) from a blood test. In contrast to existing methods that are complex and costly, the single-tube, single-step protocol detect absolute copy number alterations (CNA) in single cells and maintain accuracy at a lower cost than conventional genomic analysis procedure, opening up to the possibility for genome-driven targeted therapy selection and monitoring of disease progression in liquid biopsy.

|
Scooped by
Gilbert C FAURE
September 29, 2017 5:16 AM
|

|
Scooped by
Gilbert C FAURE
July 20, 2017 2:47 AM
|
Click here to edit the content

|
Scooped by
Gilbert C FAURE
May 28, 2017 3:30 AM
|
The Kuhn Laboratory at the University of Southern California and the Dive Laboratory at the Cancer Research UK's Manchester Institute are teaming up to apply new cancer cell detection technology t

|
Scooped by
Gilbert C FAURE
March 7, 2017 12:56 PM
|
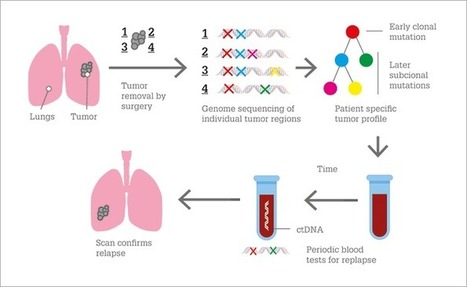
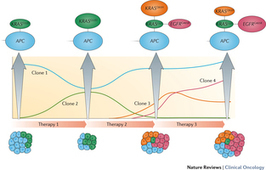
During cancer progression and treatment, multiple subclonal populations of tumour cells compete with one another, with selective pressures leading to the emergence of predominant subclones that replicate and spread most proficiently, and are least susceptible to treatment. At present, the molecular landscapes of solid tumours are established using surgical or biopsy tissue samples. Tissue-based tumour profiles are, however, subject to sampling bias, provide only a snapshot of tumour heterogeneity, and cannot be obtained repeatedly. Genomic profiles of circulating cell-free tumour DNA (ctDNA) have been shown to closely match those of the corresponding tumours, with important implications for both molecular pathology and clinical oncology. Analyses of circulating nucleic acids, commonly referred to as 'liquid biopsies', can be used to monitor response to treatment, assess the emergence of drug resistance, and quantify minimal residual disease. In addition to blood, several other body fluids, such as urine, saliva, pleural effusions, and cerebrospinal fluid, can contain tumour-derived genetic information. The molecular profiles gathered from ctDNA can be further complemented with those obtained through analysis of circulating tumour cells (CTCs), as well as RNA, proteins, and lipids contained within vesicles, such as exosomes. In this Review, we examine how different forms of liquid biopsies can be exploited to guide patient care and should ultimately be integrated into clinical practice, focusing on liquid biopsy of ctDNA — arguably the most clinically advanced approach.

|
Scooped by
Gilbert C FAURE
January 25, 2017 12:11 PM
|
The challenge to obtain needle biopsy samples from patients with cancer has steered the development of new blood-based diagnostics called 'liquid biopsy'. In 2016, major advances have been made in the use of circulating tumour cells and cell-free DNA for monitoring tumour evolution in patients with cancer of the gastrointestinal tract, with a focus on colorectal cancer.

|
Scooped by
Gilbert C FAURE
December 1, 2016 2:18 PM
|
Purpose: Recent studies demonstrate that prostate cancer clones from different metastatic sites are dynamically represented in the blood of patients over time, suggesting that the paired evaluation of tumor cells in circulation and bone marrow, the primary target for prostate cancer metastasis, may provide complementary information. Experimental Design: We adapted our single-cell high-content liquid biopsy platform to bone marrow aspirates (BMA), to concurrently identify and characterize prostate cancer cells in patients' blood and bone and thus discern features associated to tumorigenicity and dynamics of metastatic progression. Results: The incidence of tumor cells in BMAs increased as the disease advanced: 0% in biochemically-recurrent (n=52), 26% in newly diagnosed metastatic hormone-naïve (n=26), and 39% in metastatic castration-resistant (mCRPC; n=63) patients, and their number was often higher than in paired blood. Tumor cell detection in metastatic patients' BMAs was concordant but 45% more sensitive than using traditional histopathologic interpretation of core bone marrow biopsies. Tumor cell clusters were more prevalent and bigger in BMAs than in blood, expressed higher levels of the androgen receptor protein per tumor cell and were prognostic in mCRPC. Moreover, the patterns of genomic copy number variation in single tumor cells in paired blood and BMAs showed significant inter and intrapatient heterogeneity. Conclusions: Paired analysis of single prostate cancer cells in blood and bone shows promise for clinical application and provides complementary information. The high prevalence and prognostic significance of tumor cell clusters particularly in BMAs, suggest that these structures are key mediators of prostate cancer's metastatic progression.

|
Scooped by
Gilbert C FAURE
August 19, 2016 9:48 AM
|
Article Citation: Lynette M. Sholl,Dara L. Aisner,Timothy Craig Allen,Mary Beth Beasley,Philip T. Cagle,Vera L. Capelozzi,Sanja Dacic,Lida P. Hariri,Keith M. Kerr,Sylvie Lantuejoul,Mari Mino-Kenudson,Kirtee Raparia,Natasha Rekhtman,Sinchita Roy-Chowdhuri,Eric Thunnissen,Ming Tsao,Marina Vivero,Yasushi Yatabe, (2016) Liquid Biopsy in Lung Cancer: A Perspective From Members of the Pulmonary Pathology Society. Archives of Pathology & Laboratory Medicine: August 2016, Vol. 140, No. 8, pp. 825-829. doi: http://dx.doi.org/10.5858/arpa.2016-0163-SA

|
Scooped by
Gilbert C FAURE
June 14, 2016 2:05 PM
|
Vortex Biosciences provides an easy-to-use, label-free, without sample preparation, benchtop solution for routine isolation and purification of CTCs from your liquid biopsy samples
SAN DIEGO, May 31, 2016 /PRNewswire/ -- Biocept, Inc. (NASDAQ: BIOC), a molecular diagnostics company commercializing and developing liquid biopsies to improve the diagnosis and treatment of cancer, announces the expansion of its liquid biopsy offering into immuno-oncology with the commercial launch of its PD-L1 protein expression test. The CLIA-validated test uses Biocept's proprietary, patented Target Selector™ platform with circulating tumor cells (CTCs) from a patient's blood sample and can be used to detect and monitor PD-L1 protein expression throughout the course of a patient's cancer therapy. The PD-L1 test was developed by Biocept's scientists in collaboration with David Rimm, MD, PhD, Professor of Pathology and Medicine at Yale University School of Medicine and a scientific advisor to Biocept.
Immuno-oncology therapy fights cancer by stimulating a patient's immune system to directly attack tumor cells or by enhancing anti-tumor responses to man-made immune system proteins. Patients with cancers expressing the PD-L1 protein are more likely to respond to certain immuno-oncology therapeutics and several PD-L1-related immuno-oncology therapies have received U.S. Food and Drug Administration (FDA) approval. Among the recent approvals include Keytruda® (pembrolizumab) for patients with advanced non-small cell lung cancer. Many additional immuno-oncology therapies are in clinical development.
Via Krishan Maggon

|
Scooped by
Gilbert C FAURE
May 3, 2016 4:04 AM
|
Oncotarget. 2016 Apr 26. doi: 10.18632/oncotarget.9018. [Epub ahead of print]
|
 Your new post is loading...
Your new post is loading...
 Your new post is loading...
Your new post is loading...