Your new post is loading...
 Your new post is loading...

|
Rescooped by
Giuseppe Fattori
from Co-creation in health
October 21, 2017 3:25 PM
|
Most patient and consumer advocacy groups receive funds from the pharmaceutical industry, according to a new study released by the group PharmedOut. Only a handful out of 7,685 health advocacy groups in the U.S. are completely independent of pharmaceutical industry money, according to a list the group released Oct. 13. PharmedOut is a Georgetown University Medical Center project that advances evidence-based prescribing and educates health-care professionals about pharmaceutical marketing practices. And industry funding of patient groups, including websites and informational materials, is often not apparent to the average consumer, which could mislead consumers into believing they’re getting unbiased health advice. “Industry funding is often not disclosed on websites or informational materials or is hidden,” PharmedOut Director Adriane Fugh-Berman told me in an Oct. 16 phone call. Funding and sponsorship is often very subtle and difficult to identify, she said. In addition, she said, industry sponsorship can affect the stands patient and consumer groups are willing to take, she said. Groups that accept industry funding are affected by that money, regardless of whether they think they are, she said. "Look at the stands taken and not taken,” she said. “For example, where is the anger and outrage about drug costs?” Fugh-Berman is an associate professor in the Department of Pharmacology and Physiology and in the Department of Family Medicine at Georgetown University Medical Center. Further Reading:
Via Pharma Guy, Giuseppe Fattori

|
Rescooped by
Giuseppe Fattori
from Social Media and Healthcare
September 1, 2017 1:20 AM
|
Pharmaceutical companies can use social media to engage and educate patients as well as to provide reliable information about their products. But how do companies tap social media without running afoul of the Food and Drug Administration (FDA) and various regulatory requirements? To find out, I asked Colleen Tracy James, a partner in Mayer Brown’s New York office, where she is a member of the firm’s intellectual property practice and life sciences group. Belbey: What are the challenges facing pharmaceutical firms when using social media? James: The internet is the wild wild West. We are still figuring out how to maneuver and make sure information is reliable and trustworthy. This is especially important when it comes to pharmaceutical products because it impacts our health and well-being. Pharmaceutical companies face a lot of challenges on social media, such as how to handle incomplete and misinformation about their products. We all remember the famous Kim Kardashian Instagram post where she touted the benefit of a drug that treats morning sickness, but she did not disclose any of the side effects. According to the FDA Guidance, when you use social media, you need to include the ‘good, bad and the ugly' about your product. Your social media posts need to be fair and balanced. Belbey: How can pharmaceutical companies control the message and the information? James: One way to control the message is to create what the industry calls a “controlled environment” such as creating a company webpage for a drug or disease. In this way, firms can make sure information is accurate and complete. That keeps them in line with the FDA regulations. That’s very different from third party information, where the company does not have control over how its product are discussed. Belbey: Can firms use controlled environments and social media to correct misinformation? James: Yes. If patients are talking to each other and sharing a misconception of your product, you may want to, as a company, respond to that. You may decide to allow certain employees in the corporation to engage with potential consumers on social media and to answer questions. Or you could provide a chat room as part of your own platform. In that case, your obligation to correct misinformation may be heightened because now you have control of that website. Belbey: How can pharmaceutical firms use Twitter? James: Twitter is one of the areas where we’ve seen some specific guidance from the FDA. Due to its character limitations, the FDA is concerned that if you don’t have enough space, you can’t provide a fair and balanced (benefits and downsides) view of your drug. Pharmaceutical companies face serious penalties by the FDA if they don’t make the proper disclosures -- something that pharmaceutical companies want to avoid. If you are responsible for disseminating misinformation about your drug, and the wrong person takes it because he or she wasn’t aware of the contraindications or the side effects, the pharmaceutical companies open themselves up for liability. In short, pharmaceutical companies must be careful not to mislead consumers on Twitter. Belbey: Given that there are real risks associated with not complying with advertising rules and regulations, how should firms move forward safely? James: Pharmaceutical firms should develop clear and decisive social media advertising policies. First, they need to figure out which platforms they will use, how they will control their messages, whether they’re going to allow chat rooms, and how to manage comments from patients who want a dialog. If interactivity is permitted, companies need to clearly define which employees may interact with consumers, patients and doctors on social media. The firm also needs a content strategy, so that responses are in compliance with what the FDA has approved the pharmaceutical company may say about their product. You also may need a team of legal advisers to confirm that content about your product is okay to put on social media. Pharmaceutical companies must be careful that employees don’t provide people with wrong information when responding on social media. For example, you can’t say, “This medication is for a headache but you can also take it for something else that’s not a headache.” That would be a violation, historically speaking, as firms are not allowed to market their drugs’ off-label uses or mention off-label uses. And finally, I also suggest that companies have a compliance officer tasked with making sure there’s a policy in place and it’s being complied with and updated with evolving FDA guidance. Since the FDA was created, we’ve gone from radio to TV and to now social media. Just like the past when industry adapted to new forms of communications, pharmaceutical companies are now figuring out how to advertise and communicate using the latest media within the regulatory framework. Contributor’s notes: For more information, read “5 Social Media Pitfalls In The Pharmaceutical Industry” by Henniger Bullock and Colleen Tracy James, Mayer Brown LLP. https://www.mayerbrown.com/files/News/29b609ec-4b5a-4ce3-950c-04026ef73f85/Presentation/NewsAttachment/39dca070-2b87-49a6-b03a-04e8e6f9bafc/5SocialMediaPitfallsInThePharmaceuticalIndustry.pdf Here is guidance from the FDA pertaining to advertising and social media for your convenience: Medical Product Communications That Are Consistent With the FDA-Required Labeling — Questions and Answers (Draft Jan 2017): https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM537130.pdf Drug and Device Manufacturer Communications With Payors, Formulary Committees, and Similar Entities – Questions and Answers (Draft 2017): https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM537347.pdf FDA Draft Guidance for Industry Internet/Social Media Platforms with Character Space Limitations – Presenting Risk and Benefit Information for Prescription Drugs and Medical Devices (June 2014): https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm401079.pdf FDA Draft Guidance for Internet/Social Media Platforms: Correcting Independent Third-Party Misinformation About Prescription Drugs and Medical Devices (June 2014): https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm401079.pdf Fulfilling Regulatory Requirements for Postmarking Submissions of Interactive Promotional Media for Prescription Human and Animal Drugs and Biologics (Draft Jan 2014): https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm381352.pdf Responding to Unsolicited Requests for Off-Label Information About Prescription Drugs and Medical Devices (December 2011): https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm285145.pdf This blog appeared previously on Forbes.
Via Plus91

|
Scooped by
Giuseppe Fattori
March 30, 2017 5:49 AM
|
Amava viaggiare comodo e non certo a spese proprio il primario di ortopedia del Gaetano Pini di Milano, Noberto Confalonieri, da tre giorni agli arresti domiciliari per corruzione, turbativa d'asta e lesioni. Il medico era stato beccato in un'intercettazione mentre raccontava di "aver provocato la rottura del femore di un'anziana paziente di 78 anni, operata a suo dire per allenarsi con la tecnica" richiesta per impiantare protesti di due grandi aziende produttrici di materiale ortopedico.

|
Scooped by
Giuseppe Fattori
November 2, 2015 1:00 AM
|
It’s patients who lose out if doctors and professional journals stop asking the right questions. During a recent clinic consultation, I saw Mary, in her early 60s, with type 2 diabetes. She was concerned that the muscle pains in her legs may have been a result of the cholesterol-lowering statin drug she was taking. “But I’m scared of stopping it.” She explained how a specialist nurse had told her a clot could break off from her aorta, travel to her brain and cause a massive stroke. I assured her that even in those with established heart disease, who stand to gain most from taking the drug, the risk of death from stopping the medication for two weeks to see if the side-effects would go was close to 1 in 10,000 . Unfortunately, such misinformation and fear-mongering is common. One of the root causes is undoubtedly driven by the commercial interests of the pharmaceutical industry. As cardiologist eter Wilmshurst points out in a talk he gave at the Centre for Evidence-Based Medicine last year, the drug and device industry has an ethical and legal responsibility to produce profit for their shareholders but not to sell patients and doctors the best treatment. But the real scandal, he says, is the failure of regulators and the collusion of sorts between doctors, institutions and medical journals.

|
Scooped by
Giuseppe Fattori
November 1, 2015 9:32 AM
|
Amazon’s web services are now worth more than its entire retail operation – and growing three times as fast. The internet, in just two decades, has gone from something that seemed exotic to most people to a utility that is taken for granted – like electricity. Just as most people – at least in industrial societies – rarely go through a day without switching on a light, so most of us use the internet every day. In that sense, it has become what historians of technology call a GPT – a general purpose technology, like steam power, electricity, mass production and the automobile. In 1999, Andy Grove, then the CEO of Intel, was widely ridiculed for declaringthat “in five years’ time there won’t be any internet companies. All companies will be internet companies or they will be dead.” What he meant was that anybody who aspired to be in business in 2004 would have to deal with the internet in one way or another, just as they relied on electricity. And he was right; that’s what a GPT is like: it’s pervasive.

|
Scooped by
Giuseppe Fattori
October 27, 2015 3:57 PM
|
Could our relentless pursuit of good health be making us sick? Advances in medicine have propelled health care to new heights and a vast array of diagnostic tests and drug therapies is now available. But are we getting too much of a good thing? An increasing number of doctors now say that sometimes, "less is more" when it comes to medical interventions. Some doctors are concerned that resources are being wasted on the "worried well" and that the ever-expanding definition of how we define "disease" has been influenced by vested interests. Could excessive medical interventions be causing more harm than good? Dr Maryanne Demasi examines how our relentless pursuit for good health might be making us sick

|
Scooped by
Giuseppe Fattori
October 26, 2015 11:26 AM
|
Are you overpaying for your health care compared with those in a nearby city? The arbitrary pricing strategies put in place by health care providers makes large pricing swings the rule rather than the exception, experts say. It’s a puzzling (and little-known) fact of the health care industry: Health

|
Scooped by
Giuseppe Fattori
October 25, 2015 10:02 AM
|

|
Scooped by
Giuseppe Fattori
October 24, 2015 6:10 PM
|
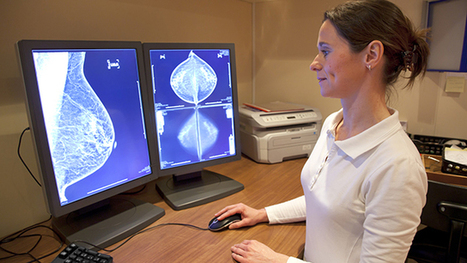
Once again, the United States Preventive Services Task Force’s latest draft report on the potential benefits and harms of mammography screening was met by outcries from radiologists and others that thousands of women would die if the recommendations were followed. The Task Force concluded that women between the ages of 50 and 74 should get mammograms every two years. But for women under 50, the chances that a mammogram will help her rather than harm her are very small. For younger women, the decision to get a mammogram should be made on a case-by-case basis. This is reasonable advice, but you wouldn’t know it from comments on Twitter and in some media outlets. In 2009, when the Task Force last issued recommendations on mammograms, everybody from breast cancer advocacy groups to members of Congress accused the panel of “killing women.” Radiologists, some of whom have a vested financial interest in mammography, claimed the panel used outdated evidence. One newspaper columnist suggested that Congress should “take pity on the Task Force and send it to the Death Panel for a humane end.” The reaction this time around has been only a little less negative.

|
Scooped by
Giuseppe Fattori
October 20, 2015 11:24 AM
|
(NaturalNews) Statins, the widely prescribed class of drugs said to lower "bad" cholesterol and reduce the risk of heart problems, has recently come under fire after a study revealed that they destroy human health more than they work to improve...

|
Scooped by
Giuseppe Fattori
May 3, 2015 12:31 PM
|
Roberto Maroni, in una conferenza stampa, ha parlato di un danno «stimato in oltre sessanta milioni di euro per il sistema sanitario regionale» La Regione Lombardia intenterà «una serie di azionilegali nei confronti dei gruppi Novartis e La Roche» per il caso del farmaco Lucentis, che sarebbe stato favorito al posto del meno caro Avastin. Lo ha annunciato il presidente Roberto Maroni che, in una conferenza stampa, ha parlato di un danno «stimato in oltre sessanta milioni di euro per il sistema sanitario regionale». Per questo la sua giunta ha deliberato di dare «incarico all’avvocatura regionale per recuperare in sede giudiziale o extragiudiziale» la somma.

|
Scooped by
Giuseppe Fattori
March 21, 2015 12:46 PM
|
Sarà di McDonald's il più grande ristorante della kermesse delle multinazionali dell'alimentazione, l'Expo. Agnoletto e Molinari: «Come se Erode fosse testimonial di Unicef»

|
Scooped by
Giuseppe Fattori
May 18, 2015 3:04 PM
|
If it often seems like America's "civil servants" and public officials no longer work for us, the people, it's because they don't. The government today, both locally and federally, has been hijacked and turned into a private corporation, including the U.S. Centers for Disease Control and Prevention (CDC), which is definitively nothing more than a for-profit business perpetuating both its own continued existence and the corporate agendas of its puppet masters.
Just to be clear, this isn't just some tongue-in-cheek rant expressing dissatisfaction with government "incompetence," or with the actions of the current political party "in charge" -- it's an undeniable fact that inevitably leads us all much further down the rabbit hole, and a truth that more Americans need to understand for the purpose of learning how to both escape from the clutches of Government Inc. and ultimately shut it down.
Certain parasitic entities, you see, have infiltrated our once-free Republic and turned it into a military-industrial fascist state that serves private interests. And you, American, if you don't learn how to "opt out" of this evil system, are a willing participant in your own tyranny, whether you realize it or not. Though it might sound complicated, it's really quite simple: The "government" that essentially rules over Americans today is an illegal, private corporation that has supplanted our real government, which once acted on behalf of the people to protect their interests and freedoms from the type of tyranny that, ironically, has taken over America from within.
Learn more: http://www.naturalnews.com/049749_CDC_United_States_corporation_Big_Pharma.html#ixzz3aWCRykVN
|

|
Scooped by
Giuseppe Fattori
September 24, 2017 12:13 PM
|
Can Pharmaceuticals Buy Doctor's Prescription Pads? Its a complicated topic but new evidence suggests it might be harder than reported in the past. Using prostate cancer drugs these investigators did not find pharmaceutical payments impact doctor's habits at all. The short answer is we found very little correlation between payments given to physicians and the amount of drug they dispense. In fact, the median amount of prescribed drugs was exactly the same between those doctors who received money from the pharmaceutical companies and those that did not. You can read the paper to get a feel for some of the other nuances of our findings but suffice it to say no strong relationship was seen. Within this narrow spectrum of prostate cancer drugs, pharma payments did not seem to make a difference at all to the prescribing habits of doctors.

|
Scooped by
Giuseppe Fattori
November 13, 2015 4:19 AM
|
Facing state budget cuts to the University of Wisconsin system and dwindling federal funding, Robert Golden would love to find a pile of money somewhere. But there are certain things Golden — and some other medical school officials across the country — say they won't do: take money from drug companies for doctor education programs and let them have any say in what goes into the courses.

|
Scooped by
Giuseppe Fattori
November 4, 2015 8:30 AM
|

|
Scooped by
Giuseppe Fattori
November 1, 2015 9:40 AM
|
Would you accept money "with no strings attached" from a robber who, in the act of stealing, happened to kill some of his victims? Would you accept money that has been stolen? Would you accept sponsorships from tobacco companies for a meeting about lung diseases? Few doctors would. Why is it then that most doctors willingly accept sponsorships from drug companies that have earned much of their money illegally while being fully aware that their criminal activities have killed thousands of patients, the very people whose interests doctors are supposed to take care of?

|
Scooped by
Giuseppe Fattori
November 1, 2015 6:50 AM
|
ObjectivesTo identify the impact of industry involvement in the publication and interpretation of meta-analyses of antidepressant trials in depression. Study Design and Setting Using MEDLINE, we identified all meta-analyses evaluating antidepressants for depression published in January 2007–March 2014. We extracted data pertaining to author affiliations, conflicts of interest, and whether the conclusion of the abstract included negative statements on whether the antidepressant(s) were effective or safe. Results We identified 185 eligible meta-analyses. Fifty-four meta-analyses (29%) had authors who were employees of the assessed drug manufacturer, and 147 (79%) had some industry link (sponsorship or authors who were industry employees and/or had conflicts of interest). Only 58 meta-analyses (31%) had negative statements in the concluding statement of the abstract. Meta-analyses including an author who were employees of the manufacturer of the assessed drug were 22-fold less likely to have negative statements about the drug than other meta-analyses [1/54 (2%) vs. 57/131 (44%); P < 0.001]. Conclusion There is a massive production of meta-analyses of antidepressants for depression authored by or linked to the industry, and they almost never report any caveats about antidepressants in their abstracts. Our findings add a note of caution for meta-analyses with ties to the manufacturers of the assessed products.

|
Scooped by
Giuseppe Fattori
October 27, 2015 2:54 AM
|
What is overuse?Overuse is a catchall term for medical tests, treatments, and other services that patients don’t need or don’t want.- Overuse occurs when a patient is hospitalized unnecessarily, or receives a test, treatment, drug, or procedure that is unnecessary, ineffective, or unwanted.
- “Unnecessary” means that a particular patient is very unlikely to benefit from the treatment because they don’t have the disease or symptom it’s intended to diagnose or treat, or because the possible harms of treatment outweigh the possible benefits.
- “Ineffective” means that the treatment has been shown to be no more effective than no treatment or a placebo (sugar pill or sham surgery).
- “Unwanted” means that the patient, if fully informed, would choose another testing or treatment option.
- Estimates of the money wasted on overuse each year range from around $200 billion to over$800 billion – between 10 percent and 30 percent of US health care spending.
- Overdiagnosis is a related problem, where patients are diagnosed with conditions that were unlikely to cause symptoms or shorten the patient’s life. With increased use of screening tests and highly sensitive imaging technologies such as MRI and CT scans, more conditions are being diagnosed based on anatomical abnormalities and treated as actual disease. This is most common for cancers like breast, prostate, and thyroid cancer, many cases of which are slow-growing and not harmful, but which many patients and doctors are uncomfortable leaving untreated. Overdiagnosis is also common for some mental health conditions, such as ADHD in children.

|
Scooped by
Giuseppe Fattori
October 25, 2015 10:13 AM
|
Risk/Reward: The Dark Money of Medicine. How drug companies funnel money to continuing medical education programs.

|
Scooped by
Giuseppe Fattori
October 25, 2015 7:44 AM
|
A review of studies that assess clinical antidepressants shows hidden conflicts of interest and financial ties to corporate drugmakers. After many lawsuits and a 2012 U.S. Department of Justice settlement, last month an independent review found that antidepressant drug Paxil (paroxetine) is not safe for teenagers. The finding contradicts the conclusions of the initial 2001 drug trial, which the manufacturer GlaxoSmithKline had funded, then used its results to market Paxil as safe for adolescents. The original trial, known as Study 329, is but one high-profile example of pharmaceutical industry influence known to pervade scientific research, including clinical trials the U.S. Food and Drug Administration requires pharma companies to fund in order to assess their products. For that reason, people who read scientific papers as part of their jobs have come to rely on meta-analyses, supposedly thorough reviews summarizing the evidence from multiple trials, rather than trust individual studies. But a new analysis casts doubt on that practice as well, finding that the vast majority of meta-analyses of antidepressants have some industry link, with a corresponding suppression of negative results.

|
Scooped by
Giuseppe Fattori
October 24, 2015 6:06 PM
|

|
Scooped by
Giuseppe Fattori
May 3, 2015 12:26 PM
|

|
Scooped by
Giuseppe Fattori
August 7, 2015 1:34 PM
|
Quelle a été l’attitude de la recherche tout au long de ces années ?
R.P.: Le tabac a représenté la plus grande atteinte à l'intégrité intellectuelle depuis l'époque nazie. Au moins 25 Prix Nobel ont perçu des sommes d'argent des fabricants, et reçu des bourses du célèbre Conseil Américain de la Recherche sur le Tabac (CTR), le principal organe responsable de la recherche menée par l'industrie pour servir d'écran de fumée. Plus de 400 millions de dollars ont été alloués à ce seul organisme et des chercheurs industriels de haut vol ont été sollicités pour participer à l'élaboration de la campagne anti-cancer menée à l'initiative de Nixon. C'est la raison pour laquelle le tabac a été quasiment passé sous silence pendant cette opération, alors que la cigarette est responsable d'environ un tiers des cancers - cancers qui n'existeraient pas sans cela.

|
Scooped by
Giuseppe Fattori
July 19, 2014 11:00 PM
|
Drug giant GlaxoSmithKline has announced it will no longer pay health care professionals to promote its products or the diseases they treat to “audiences who can prescribe or influence prescribing.” Glaxo also plans to stop compensating its sales...
|



 Your new post is loading...
Your new post is loading...