Your new post is loading...
 Your new post is loading...

|
Scooped by
Gilbert C FAURE
December 30, 2025 9:56 AM
|
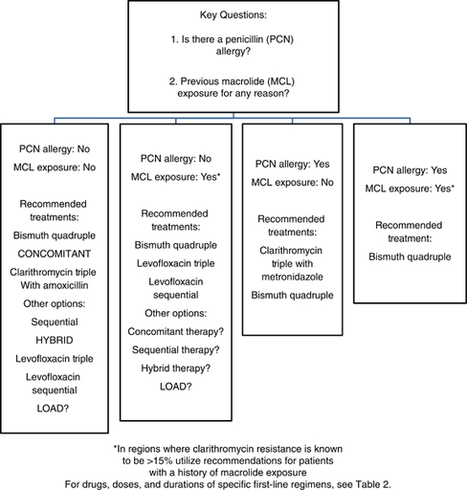
New Guidance Sets Standard for Paediatric Antibiotic Allergy Testing: in a new report, investigators USDAR-Peds introduce unified, evidence-informed protocols designed to guide clinicians in evaluating suspected antibiotic hypersensitivity in children. https://lnkd.in/dpV7Cb-S

|
Scooped by
Gilbert C FAURE
July 21, 2020 2:09 PM
|
This guideline on diagnostic procedures for suspected beta-lactam antibiotic (BLA) hypersensitivity was written by the German and Austrian professional associations for allergology, and the Paul-Ehrlich Society for Chemotherapy in a consensus procedure ...

|
Scooped by
Gilbert C FAURE
March 4, 2019 1:45 PM
|
14th International Conference on Pharmacology and Toxicology will be held during July 18-19, 2019 at Zurich, Switzerland. Pharmacology 2019 conference will focus on the theme “Research Advances in Pharmacological Field”. Pharmacology Conference focuses on the importance to understand drugs and how...

|
Scooped by
Gilbert C FAURE
December 4, 2018 4:09 AM
|
Abstract All body surfaces are exposed to a wide variety of microbes, which significantly influence immune reactivity within the host. This review provides an update on some of the critical novel findings that have been published on the influence of the microbiome on atopic dermatitis, food allergy and asthma. Microbial dysbiosis has consistently been observed in the skin, gut and lungs of patients with atopic dermatitis, food allergy and asthma, respectively, and the role of specific microbes in allergic disorders is being intensively investigated. However, many of these discoveries have yet to be translated into routine clinical practice. Abbreviations AAI allergic airway inflammation AD atopic dermatitis AHR airway hyper‐responsiveness AMP antimicrobial peptides COPD chronic obstructive pulmonary disease CRS chronic rhinosinusitis GCS glucocorticoids HDM house dust mite HMOs human milk oligosaccharides ICSs inhaled corticosteroids LABAs long‐acting β2 adrenergic receptor agonists OIT oral immunotherapy PARs protease‐activated receptors PSMs phenol‐soluble modulins RSV respiratory syncytial virus SCFAs short‐chain fatty acids TLR Toll‐like receptor 1 INTRODUCTION An enormous variety of microbes colonize the skin and mucosal body surfaces. These microbes are organized within complex community structures, utilizing nutrients from other microbes, host secretions and the diet. The microbiome is defined as the sum of these microbes, their genomic elements and interactions in a given ecological niche. In addition to bacteria, viruses are also considered to be an important component of the microbiome (virome). The composition of the microbiome is dependent on the specific body site examined, resulting in a series of unique habitats within and between individuals that can change substantially over time.1 This presents significant challenges to the local immune system, which should tolerate the presence of these microbes to avoid damaging host tissue while retaining the ability to respond appropriately to pathogens. The mechanisms that mediate host‐microbe communication are highly sophisticated and need to be constantly coordinated.2 Indeed, disrupted communication between the microbiome and the host due to altered microbiome composition and/or metabolism is thought to negatively influence immune homeostatic networks and may play a role in immune hypersensitivity to environmental exposures, such as allergens.3-5 For several years, epidemiological studies have suggested associations between the migration from traditional farming to urban environments, increase in processed food intake, lack of contact with animals and excessive hygiene practices with the increased incidence of asthma, atopic dermatitis and food allergy. However, it is only relatively recently that the importance of the gut, lung and skin microbiomes in regulation of immune tolerance and its aberrations in a variety of human diseases including allergy and asthma has been recognized.6, 7 In particular, early‐life events such as mode of delivery, breastfeeding, mother's diet and health status, antibiotics and other drug usage in pregnancy and early childhood, early‐life environment (ie, siblings, pets at home, proximity to farm animals and green areas) significantly influence the timing of bacterial colonization and establishment, which modify the risk of developing allergies and asthma, as summarized in Figure 1.8-17 In this review, we will highlight some of the recent advances in our knowledge regarding the influence of the microbiome on immune reactivity in the skin, gut and lungs of patients with atopic dermatitis, food allergy and asthma. In addition, we will discuss the potential translation and challenges associated with microbial‐based therapies in patients with these allergic disorders. 2 MICROBIOME IN ATOPIC DERMATITIS The skin microbiome is comprised of bacteria, fungi, viruses and archaeal communities, with bacteria being the most widely studied.18 The skin microbiome is influenced by age, gender, ethnicity, climate, UV exposure and lifestyle factors.19 16S ribosomal RNA (rRNA) sequencing has demonstrated that significantly diverse bacterial phyla exist on healthy skin with site‐specific differences in composition. This is primarily driven by the physiology of a skin niche. Propionibacterium species are predominantly found in sebaceous sites, with Corynebacterium and Staphylococcus species occurring in moist microenvironments. Malassezia represents the predominant fungal flora on human skin.20 Figure 2 illustrates the interactions between the skin microbiome and host cells. Atopic dermatitis (AD) is characterized by epidermal barrier dysfunction resulting from a synergistic decrease in epidermal barrier structural proteins, alteration in lipid composition and skin pH, activation of local and systemic inflammatory responses and decrease in skin microbiome diversity.19 Staphylococcus aureus overgrowth is consistently linked with AD pathogenesis and correlates with disease severity and eczematous flares.1, 21 High IL‐4 and IL‐13 levels within AD skin can deplete keratinocyte‐produced antimicrobial peptides (AMPs), cathelicidin LL‐37, human beta defensin hBD‐2 and hBD‐3, necessary for controlling pathogenic organisms.22 Defective TLR‐2 expression in Langerhans cells of AD skin has also been observed, which may contribute to the impairment in effective immune recognition and clearance of pathogenic bacteria such as S. aureus.23 Epidermal lipid composition strongly correlates with bacterial diversity and composition at typical sites for AD lesions. For example, S. aureus dominance was associated with elevated levels of ceramide AS.21 Staphylococcus aureus overgrowth with concomitant decline in Staphylococcus epidermidis is a general feature of AD and is not restricted to eczematous lesions.19, 21 Staphylococcus aureus colonization is evident in 90% of AD cases,24 associates with AD severity and increased allergen sensitization.25 Intervention studies with antimicrobials targeting S. aureus can reduce AD severity. Restoration of the epithelial barrier with anti‐inflammatory and emollient use is able to increase microbial diversity of lesional skin.1, 24 Patients with severe AD can be colonized with a single S. aureus strain, which persists even post‐eczematous flare albeit at a lower relative abundance. In contrast, S. epidermidis strains were more heterogeneous. Interestingly, patients with more severe AD were colonized with methicillin‐sensitive staphylococci, whereas less severe AD was more frequently associated with methicillin‐resistant strains. This observation may have significant treatment implications, particularly when methicillin‐sensitive S. aureus and methicillin‐resistant S. epidermidis strains are present.26 In a recent study, the skin microbiome of infants with AD showed a consistent absence of S. aureus sequences at multiple time points on lesional skin contrary to reported finding in patients with established AD. The most prevalent species were S. epidermidis and S. cohnii. However, those who developed AD at 12 months had significantly lower levels of these commensal staphylococci detectable at 2 months of age.27 This study suggests that S. aureus colonization may not always predate clinical AD and highlights the need for longitudinal studies to investigate the transition to microbial dysbiosis in AD. Commensal S. epidermidis strains can also increase during disease flares.24 Coagulase‐negative staphylococci (CoNS), which include S. epidermidis, S. hominis and S. lugdunensis, can secrete antimicrobials that limit S. aureus overgrowth and biofilm formation.1, 28 In addition, S. epidermidis activates TLR2, thereby promoting tight junction protein expression and inducing keratinocyte‐derived antimicrobial peptide secretion. Early occupation of the neonatal human skin by S. epidermidis is associated with induction of S. epidermidis‐specific FOXP3+ Treg cells that regulate local activation of host immune responses.29 Other members of the healthy skin microbiota, such as Propionibacterium, Streptococcus, Acinetobacter, Corynebacterium, Prevotella and Proteobacteria, are frequently reduced in AD patients.28, 30 Staphylococcus aureus can contribute to epidermal barrier disruption in a number of ways. Staphylococcus aureus downregulates terminal differentiation proteins such as filaggrin and loricrin, while secretion of proteases contributes to the disruption of the epidermal integrity via direct proteolytic activity or activation of protease‐activated receptors (PARs). Superantigens such as staphylococcal enterotoxins A and B or toxic shock syndrome toxin‐1 trigger a cytokine response that further disrupts the epidermal barrier. These enterotoxins also act as allergens, and toxin‐specific IgE contributes to cutaneous inflammation.28, 31 Staphylococcus aureus expresses exotoxins such as cytolytic α‐toxin, which damage keratinocytes, while β‐, γ‐ and δ‐toxins stimulate mast cell degranulation.28, 32 Phenol‐soluble modulins (PSMs) induce keratinocyte damage and secretion of the alarmins IL‐1α and IL‐36α, which further exaggerate skin inflammation.33 An impaired skin barrier results in increased exposure of the immune system to microbial components, resulting in a progressive cycle of inflammatory responses and tissue damage. It was recently suggested that reactivity to S. aureus can be facilitated via allergen co‐exposure and vice versa since patients with sensitization to house dust mite also show significantly more IgE reactivity to S. aureus and Escherichia coli, two abundant species in the house dust mite microbiome.34 A subset of AD patients is susceptible to eczema herpeticum (EH), and S. aureus may contribute to EH susceptibility as it has been shown to secrete products that enhance viral replication.1 Despite Malassezia species having a commensal role in healthy skin, in AD Malassezia may contribute to disease pathogenesis. Malassezia DNA has been detected in 90% of AD skin lesions, and colonization increases with disease severity.35 In addition, different Malassezia strains were found in AD and healthy individuals suggesting the existence of key pathogenic strains in AD.36 Higher levels of IgE sensitization to Malassezia have been detected in adult AD compared to healthy individuals and childhood AD.22, 36 Malassezia could contribute to AD pathogenesis by secreting immunogenic proteins that induce proinflammatory cytokines, expression of TLR2 and TLR4 on keratinocytes and induction of auto‐reactive T cells.22 Atopic dermatitis is considered a first step in the atopic diathesis, facilitated in part by the defective epidermal barrier of AD. The IL‐4/IL‐13 axis in AD is also thought to upregulate the pore‐forming claudin‐2 expression in the gut leading to barrier defects.19 In addition to the skin microbiota, AD has been associated with changes in the gut microbiota. Patients with AD have lower levels of Bifidobacterium in the gut compared to healthy controls, and Bifidobacterium levels were inversely correlated with AD disease severity.37 Several studies have shown that alterations in gut microbiota composition can precede the development of AD. Early gut colonization with C. difficile was associated with AD development,38 and low gut microbiota diversity and specifically low Bacteroidetes diversity at 1 month were associated with AD development at 2 years of age.35, 39 A recent whole‐metagenome analysis demonstrated a lower abundance of key metabolic pathways in AD children associated with depletion of mucin‐degrading bacteria such as Akkermansia muciniphila, Ruminococcus gnavus and Lachnospiraceae.40 These bacteria not only are able to influence immune development through directly influencing signalling pathways and antigen processing but also can lead to a reduced microbial diversity as these bacteria are able to degrade complex polysaccharides into short‐chain fatty acids (SCFAs)—nutrient sources that allow for gut colonization by other microbes.40 Dog exposure at birth was associated with a dose‐related reduced risk of AD in early life, suggesting that exposure to an environment rich in microbial components may be protective.41 In contrast, antibiotic exposure during the first 2 years of life is associated with an increased risk of AD.42 Infants with high faecal calprotectin levels (an antimicrobial protein used as a biomarker of intestinal inflammation) measured at 2 months of age had an increased risk of AD and asthma by 6 years of age. High faecal calprotectin was also shown to be inversely correlated with levels of E. coli. Reduced early colonization with E. coli was shown to impair IL‐10 regulation.43 3 MICROBIOME IN FOOD ALLERGY The human gut microbiome is increasingly being considered as a crucial factor in the development of food allergy, with a strong interrelation between the human gut microbiota, environmental factors, human genetics and gastrointestinal atopy.4, 44 In particular, the composition and metabolic activity of the gut microbiota are intimately linked with the development of oral tolerance.45, 46 Therefore, disturbed microbial homeostasis, especially early in life, appears to significantly influence allergic disease susceptibility. Figure 3 illustrates some of the known interactions between the gut microbiome and host mucosal cells. Recently, the oral bacterial composition in saliva samples from healthy and allergic children up to 7 years of age was described. The result confirmed that early changes in oral microbial composition seem to associate with immune maturation and allergy development.47 Milk‐allergic infants have higher total bacteria and anaerobic bacterial counts compared with healthy control children after 6 months of differential formula intake. In addition, higher proportions of Lactobacilli and lower proportions of Enterobacteria and Bifidobacteria were observed in 46 milk‐allergic infants.48 The spontaneous resolution of milk allergy in infants was associated with a specific gut microbiota composition.49 Bunyavanich et al showed that Clostridia and Firmicutes were enriched in the infant gut microbiome of subjects whose milk allergy spontaneously resolved. This result suggested that early infant gut microbiota may shape food allergy outcomes in childhood and bacterial taxa within Clostridia and Firmicutes species could be further investigated as probiotic candidates for milk allergy therapy.49 An additional study examining the gut microbiome of 141 children with egg allergy and healthy controls found that genera from Lachnospiraceae and Ruminococcaceae were associated with egg sensitization; however, there was no association between early‐life gut microbiota and egg allergy resolution by age 8 years.50 A prospective microbiome association study in 14 children with food allergy and 87 children with food sensitization showed that the genera Haemophilus, Dialister, Dorea and Clostridium were underrepresented among subjects with food sensitization, whereas the genera Citrobacter, Oscillospira, Lactococcus and Dorea were underrepresented among subjects with food allergy.51 An additional prospective study identified both temporal variation and long‐term variation in the differential abundance of specific bacterial genera in children developing IgE‐associated allergic disease, with Faecalibacterium correlating with IL‐10 and Foxp3 mRNA levels.52 Human milk oligosaccharides (HMOs) have been shown to be important in supporting the establishment of the infant gut microbiome as they are selective substrates for protective microbes such as Bifidobacteria.53 Two recent studies have described differences in HMO composition that are associated with cow's milk allergy or food sensitization.54, 55 One potential mechanism for this association is that different HMO profiles may support the establishment of different microbes early in life, thereby indirectly influencing immune maturation and education. In conclusion, a number of human studies now suggest that food allergy could be associated with changes in microbial exposures in early life, which modifies the development of host immunity and results in pathologic immune responses to food allergens. 4 MICROBIOME IN ASTHMA Composition of the microbiome at all mucosal sites changes dynamically in the first days, months and years of life. If the process of “healthy” and timely colonization is disrupted, the early‐life dysbiosis of the gut and lung becomes an important risk factor for atopy, allergy and asthma. In the Canadian Healthy Infant Longitudinal Development (CHILD) study, the lower relative abundance of the bacterial genera Lachnospira, Veillonella, Faecalibacterium and Rothia in the gut was associated with the development of asthma later in life and mechanistically linked with the reduced levels of faecal SCFAs.56 Another recent study also showed that high levels of SCFAs early in life were protective against later life sensitization and asthma.57 In a US birth cohort, lower relative abundance of Bifidobacterium, Akkermansia and Faecalibacterium, with higher relative abundance of Candida and Rhodotorula, in the gut of neonates significantly increased the risk of developing multisensitized atopy and asthma later in life.58 Interestingly, the faecal metabolome of those children at increased risk contained increased levels of pro‐inflammatory metabolites, among which 12, 13‐DiHOME was able to induce IL‐4 production in CD4+ T cells and decreased the abundance of Tregs.58 Increased abundance of nasopharyngeal Lactobacillus species during acute respiratory infection with respiratory syncytial virus (RSV) in infancy was associated with reduced risk of wheezing at 2 years of age.59 Colonization of the airways with Streptococcus, Moraxella or Haemophilus within the first 2 months of life was associated with virus‐induced acute respiratory infections in the first 60 weeks of life as well as increased risk of asthma later in life.60 Colonization of the hypopharynx within the first month of life with Moraxella catarrhalis, Haemophilus influenzae or Streptococcus pneumoniae was associated with low‐grade systemic inflammation as assessed by serum CRP, TNF‐alpha and IL‐6 levels.61 In addition, a positive association was observed between RSV infection and hospitalization in children with nasopharyngeal colonization with H. influenzae and Streptococcus.62, 63 Importantly, the relative nasopharyngeal abundance of Streptococcus and Staphylococcus negatively correlated with FEV1 and PC20 in children.64 Children who were breastfed and those who had low rates of respiratory infections in the first 2 years of life were colonized early within the upper respiratory tract with Staphylococcus species, followed by Corynebacterium, Dolosigranulum and Moraxella.65-67 However, the most impressive data regarding asthma protection have been observed in relation to traditional farming environments, associated with a high endotoxin and bacterial‐containing dust within the home.5, 15, 17, 68-71 Adult asthma patients treated with inhaled corticosteroids (ICSs) have greater upper and lower airway microbiota diversity compared to control subjects, especially enriched in the phylum Proteobacteria, which include Haemophilus, Comamonadaceae, Sphingomonadaceae, Nitrosomonadaceae, Oxalobacteraceae and Pseudomonadaceae families.72-76 The phylum Proteobacteria is also associated with worse asthma control, whereas Actinobacteria correlates with improvement or no change in asthma control.77 Interestingly, neutrophilic exacerbations of asthma and chronic obstructive pulmonary disease (COPD) correlated with the presence of Proteobacteria in the sputum, whereas eosinophilic exacerbations correlated with the presence of Bacteroidetes.78 Mycoplasma pneumoniae and Chlamydophila pneumoniae are also often found in the airways of the severe asthmatic.79 Macrolide antibiotic treatment may be useful in this subgroup of patients, but patients should be carefully selected.80 Both clarithromycin and azithromycin have been shown to reduce airway hyper‐responsiveness and decrease the abundance of Pseudomonas, Haemophilus and Staphylococcus,73, 81 while increasing the relative abundance of Streptococci.82 However, it is currently not clear how significant a role asthma medications play in directly influencing the composition of the airway microbiota. It has been reported that combination of ICS and oral glucocorticoids (GCS) correlates positively with the increased abundance of Proteobacteria, specifically Pseudomonas, and with a decreased abundance of Bacteroidetes, Fusobacteria and Prevotella.83 In corticosteroid‐resistant patients, Neisseria‐Haemophilus, Campylobacter and Leptotrichia species are present in the lower airways.75 Interestingly, treatment of COPD patients with ICS and long‐acting β2 adrenergic receptor agonists (LABAs), compared to LABA alone, significantly increased the bacterial load, increased bacterial diversity and changed composition of the microbiome in the airways.84 However, prospective longitudinal studies involving corticosteroid‐naïve asthma patients are still needed to address the issue of medication effects on the airway microbiome. The mechanisms responsible for changes in the airway microbiome are also not well understood, and in addition to medications, it is possible that the type of inflammatory response (ie, eosinophil vs neutrophil), changes in host secretions (eg, lipids85, 86) and cellular metabolism might influence microbial colonization and growth within the airways. Figure 4 illustrates the immune responses in the airways that can be influenced by the respiratory microbiome. In addition to asthma, the potential for microbes to play a role in the initial aetiology of rhinitis, or in exacerbations and progression to more severe inflammatory sequelae (such as asthma) is currently being examined. The phylum Proteobacteria is enriched in children with rhinitis, which may be clinically important given the Proteobacteria‐related asthma associations described above.69 Dysbiosis of the inferior turbinate mucosa microbiota, particularly an increase in S. aureus and a decrease in P. acnes, was associated with high total IgE levels in adults with allergic rhinitis.87 In adults with chronic rhinosinusitis (CRS), the genus Corynebacterium was depleted, accompanied by increased relative abundance of genera from the phyla Firmicutes (including Staphylococcus and Streptococcus), Proteobacteria (including Haemophilus, Pseudomonas and Moraxella) or Fusobacteria. This trend was particularly evident in subjects with comorbidities such as asthma and cystic fibrosis.88 Similarly, another study reported that middle meatus samples from CRS patients without nasal polyps were enriched in Streptococcus, Haemophilus and Fusobacterium but exhibited loss of diversity compared to healthy, CRS with nasal polyps and allergic rhinitis subject samples.89 5 LEARNING FROM ANIMAL MODELS Despite the compelling observations and associations in humans that link changes in the microbiota with allergic diseases, very often the causal relationship is not clear. Microbial dysbiosis can be the reason for the disease but can also be the consequence of inappropriate immune reactivity. Animal models have been used to better understand the role of microbes in directly influencing allergic diseases and to elucidate the molecular mechanisms underpinning host‐microbe crosstalk. 5.1 Atopic dermatitis Similar to humans, dogs naturally develop AD and associated allergen sensitization. Canine AD is associated with reduced bacterial diversity, with increased abundance of Staphylococcus pseudintermedius and Corynebacterium species.90 Canine AD lesions improve with antimicrobial treatment and a reduction in Staphylococcus species coincided with restoration of bacterial diversity.30 Filaggrin‐deficient flaky tail mice carry a loss‐of‐function filaggrin mutation, which is associated with a defective epidermal barrier, epidermal hydration and flexibility. Staphylococcus aureus abundance on the skin of these mice correlates with Th2 cytokine levels.91 Inbred DS‐Ng mice develop spontaneous dermatitis, and the skin lesions have been shown to be heavily colonized by S. aureus.29 Staphylococcus aureus triggered cutaneous inflammation involve the accessory gene regulatory (Agr) virulence systems of S. aureus and induced δ‐toxin molecules, which initiate Th2 type skin inflammation. Targeted S. aureus and Corynebacterium bovis antimicrobial therapy improved eczematous lesions and increased bacterial diversity in Adam 17 (a transmembrane metalloproteinase)‐deficient mice. Withdrawal of targeted antimicrobials resulted in a recurrence of eczema and microbial dysbiosis.30 In a mouse itch model, IL‐17A and IL‐22 drive neutrophils to limit the overgrowth of S. aureus on injured skin.25 C5aR‐deficient mice develop reduced microbial diversity, suggesting that the complement system may also regulate the skin microbiota.29 A mouse model of AD showed that application of a Vitreoscilla filiformis bacterial lysate reduced the inflammatory manifestations following allergen application.24 Studies in mice during the neonatal period suggest that tolerance to skin commensals such as S. epidermidis is preferentially established early in life. This supports the hypothesis that exposure to certain microbes at a critical window early in life is required for normal development of the immune system.30 5.2 Food allergy The potential role of the gut microbiome in food allergy has been studied in multiple murine models. Rodriguez et al92 demonstrated that intestinal colonization with Staphylococcus protects against oral sensitization and allergic responses. The microbiota of allergen‐sensitized IL‐4raF709 mice differentially promoted OVA‐specific IgE responses and anaphylaxis when reconstituted in wild‐type germ‐free mice, which could play a role in food allergy.93 The disease‐susceptible IL‐4raF709 mice display enhanced signalling through the interleukin‐4 receptor (IL‐4R) and exhibit STAT6‐dependent impaired generation and function of mucosal allergen‐specific Treg cells, which failed to suppress mast cell activation and expansion.94 Interestingly, STAT6 gene variants are also implicated in the pathophysiology of food allergy in humans.95 The gut microbiota can also regulate Th2 responses through the induction of RORγt Treg cells and Th17 cells.96 Certain bacterial strains such as Bifidobacterium longum 35624, Lactobacillus rhamnosus JB‐1, Clostridia species and Bacteroides fragilis can induce intestinal Treg cells that are able to suppress food allergy and colitis.97, 98 Pattern‐recognition receptor activation on DCs is a potential mechanism by which intestinal microbes may promote Treg cell differentiation.99 5.3 Asthma Important insights regarding the role of the microbiota in the pathogenesis of airway inflammation have come from mouse models. Neonatal mice are more susceptible to develop house dust mite (HDM)‐induced allergic airway inflammation (AAI) and airway hyper‐responsiveness (AHR) than mature mice.100 This phenomenon was associated with a shift from Gammaproteobacteria and Firmicutes towards a Bacteroidetes‐dominated microbiota and the development of PDL‐1–dependent Helios‐ Treg cells.100 Mice housed under germ‐free conditions display significantly more pronounced type 2 inflammation and AHR as compared to conventionally colonized mice. Recolonization, especially early in life, can reverse many of these immunological defects.101 Similarly, antibiotic‐driven dysbiosis in neonatal mice leads to impaired maturation of Tregs and enhanced Th2 responses and promotes proinflammatory colonic iNKT cells.80, 102-105 Conversely, specific bacterial strains, their components or metabolites can successfully induce a variety of anti‐inflammatory responses in the gut and in the lung. L. rhamnosus decreased AAI and AHR induced by Bet v 1 in mice.106 Bacterial strains isolated from neonatal mouse lungs and then administered intranasally very early in life (starting at day 2 after birth) can protect or worsen HDM‐induced airway inflammation, depending which cytokine profile they induced in vitro on precision‐cut lung slices.107 Intramuscular treatment with a DNA plasmid encoding a M. leprae 65 kDa heat‐shock protein (DNA‐HSP65) or subcutaneous injections with proteins from M. tuberculosis delivered in the presence of the TLR9 agonist CpG were able to significantly inhibit development of Der p 1‐induced AAI and AHR in MyD88‐ or Fas‐dependent manner.108 In addition, an exopolysaccharide from B. longum subsp. longum 35624 was shown to protect against colitis and AAI in murine models, which was dependent on TLR2‐induced IL‐10 secretion.109, 110 SCFAs or dietary fibres that are metabolized to SCFAs potently reduced experimental asthma, as well as increased the levels of colonic Bacteroidetes and Actinobacteria species, while decreasing the levels of Firmicutes and Proteobacteria.111, 112 Importantly, the beneficial effects of SCFAs or a high‐fibre diet were transferred to the offspring after treatment of pregnant mice via epigenetic mechanisms.112, 113 Mechanistically, SCFAs have been repeatedly shown to increase Treg numbers and effectiveness.114, 115 In addition, SCFAs influence bone marrow haematopoiesis,111 reduce effector T‐cell activity,116 improve epithelial barrier117, 118 and inhibit mast cell and ILC2 activation.119, 120 Other bacterial metabolites, such as histamine, can induce a wide and complex spectrum of regulatory mechanisms.121, 122 Increased numbers of histamine‐secreting bacteria were observed in adult patients with asthma and correlated with asthma severity.123 Histamine signalling through the H2R is involved in AAI,124 while the use of H2R antagonists in children during their first 6 months of life is associated with significantly increased risk of allergic diseases and asthma.10 6 THERAPEUTIC TARGETING OF THE MICROBIOME Despite the growing number of studies that associate changes in the microbiota with allergic and immune‐related outcomes, only a relatively small number of studies have shown clinical benefits and there are no microbe‐based therapies that are currently universally accepted for the prevention or treatment of allergies or asthma. A number of reasons can be suggested for this, which may include the poor choice of therapeutic microbes to begin with. It is likely that many confounding factors do influence the success of a microbiome therapeutic, such as diet, age, obesity, ethnicity and other environmental exposures. These need to be taken into account and controlled for. In addition, given the explosion in knowledge regarding disease endotypes, it is possible that specific microbes will need to be carefully selected to mechanistically fit with specific disease endotypes and it is likely that one intervention will not work for everyone. Certain interventions such as faecal transplantation may be too crude an approach, and until critical safety concerns are resolved, this type of intervention should not be considered outside the setting of carefully monitored clinical trials. 6.1 Atopic dermatitis Early intervention aimed at protecting the skin barrier may ameliorate progression of the atopic march in a subset of patients.19 Skin microbiome manipulation may offer novel therapeutic opportunities, as has been seen with the emollients supplemented with a Vitreoscilla filiformis lysate.125 Similarly, topically administration of Roseomonas mucosa improved clinical severity scores in adults and children with AD.125 Autologous microbiome transplant (AMT) of S. hominis and S. epidermidis showed efficacy in controlling S. aureus overgrowth.126 In addition to topical bacterial treatments, oral administration of probiotics has also been examined. Prenatal and post‐natal treatment with Lactobacillus and Bifidobacterium strains can reduce risk of AD development in infants,35, 127, 128 which may associate with changes in T cell–mediated responses.129 A mixture of probiotic strains was recently shown to reduce SCORAD index and topical steroid use in children with AD.130 Little has been reported on probiotic treatment of adults with AD, but administration of B. longum 35624 to adults with psoriasis resulted in reduced circulating CRP, TNF and IL‐17 levels, possibly due to increased numbers of Tregs, which suggests that bacteria in the gut can influence skin inflammatory activity in adults.131, 132 Taken together, supplementation with specific probiotic strains may modulate the gut bacteria in a way that influences inflammation within the skin and may protect some children against AD development.35 6.2 Food allergy The use of probiotics in food allergy treatment and prevention has been examined. Supplementation of cow's milk‐allergic children with Lactobacillus casei and Bifidobacterium lactis did not accelerate cow's milk allergy resolution.133 However, the combination of L. rhamnosus GG and extensively hydrolysed casein formula did accelerate milk allergy resolution after 6 and 12 months when compared to the formula‐only control group.134 The combination of L. rhamnosus supplementation and peanut oral immunotherapy (OIT) was evaluated in peanut‐allergic children for 18 months. The combination was effective in inducing possible sustained unresponsiveness and immune changes that suggested modulation of the peanut‐specific immune response.135 In addition, a sustained beneficial effect on psychosocial impact of food allergy at 3 and 12 months after end of treatment was recently reported.136 However, the major limitation of this study is that further work is required to determine the relative contributions of the probiotic vs OIT due to the lack of an OIT and L. rhamnosus supplementation control groups in this trial. 6.3 Asthma A significant number of studies have examined the effect of probiotic supplementation on asthma‐related outcomes. A recent systematic review of probiotic studies in children with asthma identified eleven studies eligible with a total of 910 children. The proportion of children with fewer episodes of asthma was significantly higher in the probiotic group than in the control group, but no statistical significance was observed in childhood asthma control test, asthmatic symptom in the day and night, the number of symptom‐free days, forced expiratory volume in the first second predicted and peak expiratory flow.137 In the future, it will be interesting to evaluate microbial administration directly to the airways, in addition to the gut.138 7 CONCLUSIONS Significant advances have been made in recent years in describing the composition of the microbiome in the gut, airways and skin. The changes in bacterial communities that associate with, or sometimes precede, atopic dermatitis, food allergy and asthma are being identified (summarized in Table 1). Accumulating evidence suggests that microbial exposures might be most effective at preventing atopic disorders during the first 1‐2 years of life. However, substantial gaps in our knowledge on the microbiome still exist. In particular, the field has been slow to translate potentially effective microbiome‐associated therapies into the clinic via appropriate clinical trials performed to high standards and showing meaningful clinical responses that are superior to current avoidance approaches. While the critical role of the microbiota in cancer immunotherapy has been established, there are currently no published data on the potential role of the microbiota in influencing the success of immunotherapy or biologics in allergy or asthma.139 In addition, novel probiotics and not just the traditional probiotic strains need to be clinically tested. Furthermore, microbial components or their metabolites should also be examined; in particular, the application of these novel microbial drugs to the diseased site (eg, the airways) must be explored. Lastly, there are no microbial therapeutics currently approved for routine clinical practice, and significant effort and investment are still required to identify the optimal microbial interventions for allergy and asthma. Location Phyll (Genus) Effect Reference Oral cavity ↑ Gemella haemolysans ↓ Lactobacillus gasseri, Lactobacillus crispatus Increased risk of allergic diseases 47 Intestine ↑ Staphylococcus species Protection against oral sensitization and allergic responses 92 Intestine ↑ Clostridia, Firmicutes Milk allergy resolution 49 Intestine ↑ Lachnospiraceae, Ruminococcaceae Associated with egg allergy 50 Intestine ↓ Haemophilus, Dialister, Dorea, Clostridium Associated with food sensitization 51 Intestine ↓ Citrobacter, Oscillospira, Lactococcus, Dorea Associated with food allergy 51 Intestine ↓ Escherichia coli High faecal calprotectin, impaired IL‐10 activation, increased risk of AD and asthma 43 Intestine ↓ Bifidobacterium Correlates with AD severity 37 Intestine Early colonization with C. difficile Associated with AD development 38 Intestine ↓ Bacteroidetes diversity Associated with AD development 39 Intestine ↓ Akkermansia muciniphila, Ruminococcus gnavus and Lachnospiraceae Associated with AD development 40 Intestine ↓ Lachnospira, Veillonella, Faecalibacterium, Rothia Reduced levels of faecal SCFAs, increased risk of asthma 56 Intestine ↓ Bifidobacterium, Akkermansia, Faecalibacterium ↑ Candida, Rhodotorula Increased risk of developing multisensitized atopy, increased circulating proinflammatory metabolites 58 Upper airways Early colonization with Staphylococcus species, Corynebacterium, Dolosigranulum, Moraxella Associated with lower rate of respiratory infections in the first 2 years of life 65-67 Upper airways Early colonization with Streptococcus, Moraxella, Haemophilus Increased risk of virus‐induced acute respiratory infections and increased risk of asthma 60 Upper airways ↑ Proteobacteria Associated with rhinitis in children 69 Nasopharynx ↑ Haemophilus influenzae, Streptococcus species Increased risk of hospitalization during RSV infection 62 Nasopharynx Colonization with Staphylococcus aureus Decreased risk of hospitalization during RSV infection 63 Nasopharynx ↑ Streptococcus, Staphylococcus Abnormalities in functional tests of the respiratory system 64 Nasopharynx ↑ Staphylococcus aureus ↓ P. acnes Associated with high IgE levels 87 Nasopharynx ↑ Firmicutes (Staphylococcus & Streptococcus), Proteobacteria (Haemophilus, Pseudomonas & Moraxella), Fusobacteria ↓ Corynebacterium Associated with CRS in adults 88 Nasopharynx ↑ Streptococcus, Haemophilus, Fusobacteria ↓ Diversity Associated with CRS without nasal polyps in adults 89 Nasopharynx ↑ Lactobacillus during acute respiratory infection with RSV Reduced risk of wheezing 59 Hypopharynx Colonization with Moraxella catarrhalis, Haemophilus influenzae, Streptococcus pneumoniae Low‐grade systemic inflammation 61 Lower airways ↑ Proteobacterium (Klebsiella species) (Mycoplasma pneumoniae, Chlamydophila pneumoniae) Associated with severe asthma 77, 79 Lower airways ↑ Actinobacteria Improvement in asthma control 77 Lower airways ↑ Neisseria, Haemophilus, Campylobacter, Leptotrichia Associated with resistance to corticosteroids in asthma 75 Sputum ↑ Proteobacteria Associated with neutrophilic asthma exacerbations 78 Sputum ↑ Bacteroidetes Associated with eosinophilic asthma exacerbations 78 Skin ↑ Staphylococcus aureus Epidermal barrier dysfunction, cutaneous inflammation, formation of AD skin lesions, associated with AD severity and allergen sensitization, associated with susceptibility to eczema herpeticum among AD patients 19, 21, 23 Skin Colonization with single clonal Staphylococcus aureus strains Associated with AD severity 26 Skin ↑ Malassezia species. Associated with AD severity 35 Skin ↑ Corynebacterium, Proteobacterium Associated with AD severity 21 Skin ↑ coagulase‐negative staphylococci: (Staphylococcus epidermidis, S. hominis, S. lugdunensis) Limits Staphylococcus aureus overgrowth 28 Skin Colonization with S. epidermidis TLR2 activation, epidermal barrier maintenance 1 Skin ↓ Proteobacteria (Propionibacterium, Streptococcus, Acinetobacter, Corynebacterium, Prevotella) Associated with AD 28, 30 Skin Early colonization with S. epidermidis Local activation of the host immune response through induction of S. epidermidis‐specific FOXP3 Treg cells 29 Skin ↑ in resident skin bacteria Associated with AD flares 24 This table summarizes the bacterial changes that have been associated with atopic dermatitis, food allergy or asthma. CONFLICTS OF INTEREST LOM is a consultant to Alimentary Health Ltd and has received research funding from GSK. NL, PS, ZL, MS and TE have no conflict of interest in relation to this work. AUTHOR CONTRIBUTIONS NL, PS, ZL, MS, TE and LOM contributed to drafting the manuscript. All authors read, reviewed and agreed the final version of this manuscript. REFERENCES Notes : This table summarizes the bacterial changes that have been associated with atopic dermatitis, food allergy or asthma.

|
Scooped by
Gilbert C FAURE
July 23, 2018 2:27 PM
|
![Figure][1]
Allergic asthma is a chronic inflammatory disease primarily mediated by Th2 immune mechanisms. Numerous studies have suggested that early life exposure to lipopolysaccharide (LPS) is negatively associated with allergic asthma. One proposed mechanism invokes desensitization of lung epithelial cells by LPS. We report here that acyloxyacyl hydrolase (AOAH), a host lipase that degrades and inactivates LPS, renders mice more susceptible to house dust mite (HDM)–induced allergic asthma. Lung epithelial cells from Aoah−/− mice are refractory to HDM stimulation, decreasing dendritic cell activation and Th2 responses. Antibiotic treatment that diminished commensal LPS-producing bacteria normalized Aoah−/− responses to HDM, while giving LPS intrarectally ameliorated asthma. Aoah−/− mouse feces, plasma, and lungs contained more bioactive LPS than did those of Aoah+/+ mice. By inactivating commensal LPS, AOAH thus prevents desensitization of lung epithelial cells. An enzyme that prevents severe lung inflammation/injury in Gram-negative bacterial pneumonia has the seemingly paradoxical effect of predisposing to a Th2-mediated airway disease.
[1]: pending:yes

|
Scooped by
Gilbert C FAURE
July 21, 2017 1:56 PM
|
Atopic dermatitis (AD) is a common inflammatory skin disease affecting up to 20% of children and 3% of adults worldwide and is associated with dysregulation of the skin barrier. Although type 2 responses are implicated in AD, emerging evidence indicates a potential role for the IL-17A signaling axis in AD pathogenesis. In this study we show that in the filaggrin mutant mouse model of spontaneous AD, IL-17RA deficiency ( Il17ra−/− ) resulted in severe exacerbation of skin inflammation. Interestingly, Il17ra−/− mice without the filaggrin mutation also developed spontaneous progressive skin inflammation with eosinophilia, as well as increased levels of thymic stromal lymphopoietin (TSLP) and IL-5 in the skin. Il17ra−/− mice have a defective skin barrier with altered filaggrin expression. The barrier dysregulation and spontaneous skin inflammation in Il17ra−/− mice was dependent on TSLP, but not the other alarmins IL-25 and IL-33. The associated skin inflammation was mediated by IL-5–expressing pathogenic effector Th2 cells and was independent of TCRγδ T cells and IL-22. An absence of IL-17RA in nonhematopoietic cells, but not in the hematopoietic cells, was required for the development of spontaneous skin inflammation. Skin microbiome dysbiosis developed in the absence of IL-17RA, with antibiotic intervention resulting in significant amelioration of skin inflammation and reductions in skin-infiltrating pathogenic effector Th2 cells and TSLP. This study describes a previously unappreciated protective role for IL-17RA signaling in regulation of the skin barrier and maintenance of skin immune homeostasis.

|
Scooped by
Gilbert C FAURE
December 21, 2015 4:14 AM
|
The prevalence and impact of #antibiotic allergies and adverse drug reactions at an Australian tertiary centre https://t.co/R2WBn4ve0D

|
Scooped by
Gilbert C FAURE
September 5, 2014 10:04 AM
|
Anaphylactic reaction linked to fruit antibiotic
Healio
She had a history of asthma and allergic rhinitis, with known anaphylaxis to penicillin and IgE-mediated cow's milk allergy.

|
Scooped by
Gilbert C FAURE
March 25, 2014 10:11 AM
|
Daily Mail Millions are told they've an allergy - but the tests are often wrong Daily Mail But a study published in the Journal of Allergy and Clinical Immunology last year found that less than 1 per cent of children thought to have an antibiotic...

|
Scooped by
Gilbert C FAURE
December 25, 2013 2:16 PM
|
RT @PROA_HULP: Antibiotic Hypersensitivity Reactions and Approaches to Desensitization http://t.co/jnJxTGmQPD [CID]

|
Scooped by
Gilbert C FAURE
June 21, 2013 1:47 AM
|
1 Section of Clinical Immunology and Allergy, Department of Pediatrics, Alberta Children's Hospital and University of Calgary, 2888 Shaganappi Trail NW, Calgary, Alberta T3B 6A8, Canada. 2 Division of Clinical Immunology and Allergy, ...
|

|
Scooped by
Gilbert C FAURE
December 22, 2025 6:47 AM
|
This is one paper to bookmark. This group is the international leader in antimicrobial allergy stewardship. Structured approach to delabeling and/or referral combined with simple and effective tools.

|
Scooped by
Gilbert C FAURE
March 9, 2019 5:40 AM
|
Researchers have identified a gene that increases the risk for a severe and potentially life-threatening reaction to the commonly prescribed antibiotic vancomycin.

|
Scooped by
Gilbert C FAURE
February 14, 2019 5:31 AM
|
Environmental exposures interplay with human host factors to promote the development and progression of allergic diseases. The worldwide prevalence of allergic disease is rising as a result of complex gene-environment interactions that shape the immune system and host response. Research shows an association between the rise of allergic diseases and increasingly modern Westernized lifestyles, which are characterized by increased urbanization, time spent indoors, and antibiotic usage. These environmental changes result in increased exposure to air and traffic pollution, fungi, infectious agents, tobacco smoke, and other early-life and lifelong risk factors for the development and exacerbation of asthma and allergic diseases. It is increasingly recognized that the timing, load, and route of allergen exposure affect allergic disease phenotypes and development. Still, our ability to prevent allergic diseases is hindered by gaps in understanding of the underlying mechanisms and interaction of environmental, viral, and allergen exposures with immune pathways that impact disease development. This Review highlights epidemiologic and mechanistic evidence linking environmental exposures to the development and exacerbation of allergic airway responses.

|
Scooped by
Gilbert C FAURE
September 18, 2018 12:05 PM
|
Download PDF William D. Chey, MD, FACG1, Grigorios I. Leontiadis, MD, PhD2, Colin W. Howden, MD, FACG3 and Steven F. Moss, MD, FACG4 1Division of Gastroenterology, University of Michigan Health System, Ann Arbor, Michigan, USA; 2Division of Gastroenterology, McMaster University, Hamilton, Ontario, Canada; 3Division of Gastroenterology, University of Tennessee Health Science Center, Memphis, Tennessee, USA; and 4Division of Gastroenterology, Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA Am J Gastroenterol 2017; 112: 212–238; doi:10.1038/ajg.2016.563; published online 10 January 2017 Received 28 June 2016; accepted 7 October 2016 Correspondence: William D. Chey, MD, FACG, Timothy T. Nostrant Professor of Gastroenterology and Nutrition Sciences, Division of Gastroenterology, University of Michigan Health System, 3912 Taubman Center, SPC 5362, Ann Arbor, Michigan 49109-5362, USA. E-mail: wchey@umich.edu Abstract Helicobacter pylori (H. pylori) infection is a common worldwide infection that is an important cause of peptic ulcer disease and gastric cancer. H. pylori may also have a role in uninvestigated and functional dyspepsia, ulcer risk in patients taking low-dose aspirin or starting therapy with a non-steroidal anti-inflammatory medication, unexplained iron deficiency anemia, and idiopathic thrombocytopenic purpura. While choosing a treatment regimen for H. pylori, patients should be asked about previous antibiotic exposure and this information should be incorporated into the decision-making process. For first-line treatment, clarithromycin triple therapy should be confined to patients with no previous history of macrolide exposure who reside in areas where clarithromycin resistance amongst H. pylori isolates is known to be low. Most patients will be better served by first-line treatment with bismuth quadruple therapy or concomitant therapy consisting of a PPI, clarithromycin, amoxicillin, and metronidazole. When first-line therapy fails, a salvage regimen should avoid antibiotics that were previously used. If a patient received a first-line treatment containing clarithromycin, bismuth quadruple therapy or levofloxacin salvage regimens are the preferred treatment options. If a patient received first-line bismuth quadruple therapy, clarithromycin or levofloxacin-containing salvage regimens are the preferred treatment options. Details regarding the drugs, doses and durations of the recommended and suggested first-line and salvage regimens can be found in the guideline. Introduction Helicobacter pylori infection remains one of the most common chronic bacterial infections affecting humans. Since publication of the last American College of Gastroenterology (ACG) Clinical Guideline in 2007, significant scientific advances have been made regarding the management of H. pylori infection. The most significant advances have been made in the arena of medical treatment. Thus, this guideline is intended to provide clinicians working in North America with updated recommendations on the treatment of H. pylori infection. For the purposes of this document, we have defined North America as the United States and Canada. Whenever possible, recommendations are based upon the best available evidence from the world’s literature with special attention paid to literature from North America. When evidence from North America was not available, recommendations were based upon data from international studies and expert consensus. This guidance document was developed using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) system (1), which provides a level of evidence and strength of recommendation for statements developed using the PICO (patient population, intervention or indicator assessed, comparison group, outcome achieved) format. At the start of the guideline development process, the authors developed PICO questions relevant to Helicobacter pylori infection. The authors worked with research methodologists from McMaster University to conduct focused literature searches to provide the best available evidence to address the PICO questions. Databases searched included MEDLINE, EMBASE and Cochrane CENTRAL from 2000 to 11 September 2014. Search terms included “pylori, treat*, therap*, manag*, eradicat*”. The full literature search strategy is provided as Supplementary Appendix 1 online. After assessing the risk of bias, indirectness, inconsistency, and imprecision, the level of evidence for each recommendation was reported as “high” (further research is unlikely to change the confidence in the estimate of effect), “moderate” (further research would be likely to have an impact on the confidence in the estimate of effect), “low” (further research would be expected to have an impact on the confidence in the estimate of effect), or “very low” (any estimate of effect is very uncertain). The strength of recommendations was determined to be “strong” or “conditional” based on the quality of evidence, the certainty about the balance between desirable and undesirable effects of the intervention, the certainty about patients’ values and preferences, and the certainty about whether the recommendation represents a wise use of resources. A summary of the recommendation statements for this management guideline is provided in Table 1. The justification for the assessments of the quality of evidence for each statement can be found in Supplementary Appendix 2 online. Table 1. Recommendation statements What is known about the epidemiology of H. pylori infection in North America? Which are the high risk groups? H. pylori infection is chronic and is usually acquired in childhood. The exact means of acquisition is not always clear. The incidence and prevalence of H. pylori infection are generally higher among people born outside North America than among people born here. Within North America, the prevalence of the infection is higher in certain racial and ethnic groups, the socially disadvantaged, and people who have immigrated to North America (Factual statement, low quality of evidence). What are the indications to test for, and to treat, H. pylori infection? Since all patients with a positive test of active infection with H. pylori should be offered treatment, the critical issue is which patients should be tested for the infection (strong recommendation; quality of evidence not applicable). All patients with active peptic ulcer disease (PUD), a past history of PUD (unless previous cure of H. pylori infection has been documented), low-grade gastric mucosa-associated lymphoid tissue (MALT) lymphoma, or a history of endoscopic resection of early gastric cancer (EGC) should be tested for H. pylori infection. Those who test positive should be offered treatment for the infection (Strong recommendation; quality of evidence: high for active or history of PUD, low for MALT lymphoma, low for history of endoscopic resection of EGC). In patients with uninvestigated dyspepsia who are under the age of 60 years and without alarm features, non-endoscopic testing for H. pylori infection is a consideration. Those who test positive should be offered eradication therapy (conditional recommendation; quality of evidence: high for efficacy, low for the age threshold). When upper endoscopy is undertaken in patients with dyspepsia, gastric biopsies should be taken to evaluate for H. pylori infection. Infected patients should be offered eradication therapy (strong recommendation; high quality of evidence). Patients with typical symptoms of gastroesophageal reflux disease (GERD) who do not have a history of PUD need not be tested for H. pylori infection. However, for those who are tested and found to be infected, treatment should be offered, acknowledging that effects on GERD symptoms are unpredictable (strong recommendation; high quality of evidence). In patients taking long-term, low-dose aspirin, testing for H. pylori infection could be considered to reduce the risk of ulcer bleeding. Those who test positive should be offered eradication therapy to reduce the risk of ulcer bleeding (conditional recommendation; moderate quality of evidence). Patients initiating chronic treatment with a non-steroidal anti-inflammatory drug (NSAID) should be tested for H. pylori infection. Those who test positive should be offered eradication therapy (Strong recommendation; Moderate quality of evidence). The benefit of testing and treating H. pylori in a patient already taking an NSAID remains unclear (conditional recommendation; low quality of evidence). Patients with unexplained iron deficiency anemia despite an appropriate evaluation should be tested for H. pylori infection. Those who test positive should be offered eradication therapy (conditional recommendation; low quality of evidence). Adults with idiopathic thrombocytopenic purpura (ITP) should be tested for H. pylori infection. Those who test positive should be offered eradication therapy (conditional recommendation; very low quality of evidence). There is insufficient evidence to support routine testing for and treatment of H. pylori in asymptomatic individuals with a family history of gastric cancer or patients with lymphocytic gastritis, hyperplastic gastric polyps, and hyperemesis gravidarum (no recommendation; very low quality of evidence). What are evidence-based first-line treatment strategies for providers in North America? Patients should be asked about any previous antibiotic exposure(s) and this information should be taken into consideration when choosing an H. pylori treatment regimen (conditional recommendation; moderate quality of evidence). Clarithromycin triple therapy consisting of a PPI, clarithromycin, and amoxicillin or metronidazole for 14 days remains a recommended treatment in regions where H. pylori clarithromycin resistance is known to be <15% and in patients with no previous history of macrolide exposure for any reason (Conditional recommendation; low quality of evidence (for duration: moderate quality of evidence)). Bismuth quadruple therapy consisting of a PPI, bismuth, tetracycline, and a nitroimidazole for 10–14 days is a recommended first-line treatment option. Bismuth quadruple therapy is particularly attractive in patients with any previous macrolide exposure or who are allergic to penicillin (strong recommendation; low quality of evidence). Concomitant therapy consisting of a PPI, clarithromycin, amoxicillin and a nitroimidazole for 10–14 days is a recommended first-line treatment option (strong recommendation; low quality of evidence (for duration: very low quality of evidence)). Sequential therapy consisting of a PPI and amoxicillin for 5–7 days followed by a PPI, clarithromycin, and a nitroimidazole for 5–7 days is a suggested first-line treatment option (conditional recommendation; low quality of evidence (for duration: very low quality of evidence)). Hybrid therapy consisting of a PPI and amoxicillin for 7 days followed by a PPI, amoxicillin, clarithromycin and a nitroimidazole for 7 days is a suggested first-line treatment option (conditional recommendation; low quality of evidence (For duration: very low quality of evidence)). Levofloxacin triple therapy consisting of a PPI, levofloxacin, and amoxicillin for 10–14 days is a suggested first-line treatment option (conditional recommendation; low quality of evidence (For duration: very low quality of evidence)). Fluoroquinolone sequential therapy consisting of a PPI and amoxicillin for 5–7 days followed by a PPI, fluoroquinolone, and nitroimidazole for 5–7 days is a suggested first-line treatment option (conditional recommendation; low quality of evidence (for duration: very low quality of evidence)). What factors predict successful eradication when treating H. pylori infection? The main determinants of successful H. pylori eradication are the choice of regimen, the patient’s adherence to a multi-drug regimen with frequent side-effects, and the sensitivity of the H. pylori strain to the combination of antibiotics administered (Factual statement; moderate quality of evidence). What do we know about H. pylori antimicrobial resistance in the North America? Data regarding antibiotic resistance among H. pylori strains from North America remains scarce. Organized efforts are needed to document local, regional, and national patterns of resistance in order to guide the appropriate selection of H. pylori therapy (strong recommendation; low quality of evidence). What methods can be used to evaluate for H. pylori antibiotic resistance and when should testing be performed? Although H. pylori antimicrobial resistance can be determined by culture and/or molecular testing, (strong recommendation; moderate quality of evidence), these tests are currently not widely available in the United States. Should we test for teatment success after H. pylori eradication therapy? Whenever H. pylori infection is identified and treated, testing to prove eradication should be performed using a urea breath test, fecal antigen test or biopsy-based testing at least 4 weeks after the completion of antibiotic therapy and after PPI therapy has been withheld for 1–2 weeks. (Strong recommendation; Low quality of evidence (for the choice of methods to test for eradication: Moderate quality of evidence)). When first-line therapy fails, what are the options for salvage therapy? In patients with persistent H. pylori infection, every effort should be made to avoid antibiotics that have been previously taken by the patient (unchanged from previous ACG guideline (1)) (Strong recommendation; moderate quality of evidence). Bismuth quadruple therapy or levofloxacin salvage regimens are the preferred treatment options if a patient received a first-line treatment containing clarithromycin. Selection of best salvage regimen should be directed by local antimicrobial resistance data and the patient’s previous exposure to antibiotics (Conditional recommendation; for quality of evidence see individual statements below). Clarithromycin or levofloxacin-containing salvage regimens are the preferred treatment options, if a patient received first-line bismuth quadruple therapy. Selection of best salvage regimen should be directed by local antimicrobial resistance data and the patient’s previous exposure to antibiotics (Conditional recommendation; for quality of evidence see individual statements below). The following regimens can be considered for use as salvage treatment: Bismuth quadruple therapy for 14 days is a recommended salvage regimen. (Strong recommendation; low quality of evidence) Levofloxacin triple regimen for 14 days is a recommended salvage regimen. (Strong recommendation; moderate quality of evidence (For duration: low quality of evidence) Concomitant therapy for 10–14 days is a suggested salvage regimen. (conditional recommendation; very low quality of evidence) Clarithromycin triple therapy should be avoided as a salvage regimen. (conditional recommendation; low quality of evidence) Rifabutin triple regimen consisting of a PPI, amoxicillin, and rifabutin for 10 days is a suggested salvage regimen (conditional recommendation; moderate quality of evidence (For duration: very low quality of evidence)). High-dose dual therapy consisting of a PPI and amoxicillin for 14 days is a suggested salvage regimen (conditional recommendation; low quality of evidence (For duration: very low quality of evidence)). When should penicillin allergy testing be considered in patients with H. pylori infection? Most patients with a history of penicillin allergy do not have true penicillin hypersensitivity. After failure of first-line therapy, such patients should be considered for referral for allergy testing since the vast majority can ultimately be safely given amoxicillin-containing salvage regimens (strong recommendation; Low quality of evidence). Question 1: What Is Known About the Epidemiology of H. pylori Infection in North America? Which Are the High-Risk Groups? Recommendation H. pylori infection is chronic and is usually acquired in childhood. The exact means of acquisition is not always clear. The incidence and prevalence of H. pylori infection are generally higher among people born outside North America than among people born here. Within North America, the prevalence of the infection is higher in certain racial and ethnic groups, the socially disadvantaged, and people who have immigrated to North America (factual statement, low quality of evidence). H. pylori infection is usually acquired during childhood (2, 3, 4, 5, 6) although the exact means of acquisition is not always clear. Risk factors for acquiring the infection include low socioeconomic status (6, 7, 8) increasing number of siblings (9) and having an infected parent—especially an infected mother (10). In the Ulm (Germany) Birth Cohort Study, the odds ratio (OR) for acquiring H. pylori infection if a child’s mother was infected was 13.0 (95% confidence interval (CI) 3.0–55.2) (10) Apart from intra-familial spread, the infection may also be transmitted through contaminated water supplies (11) particularly in developing countries. Although infection rates for male and female children are similar (3, 12) there may be a slight male preponderance of the infection in adulthood. In a meta-analysis of observational, population-based studies, men were slightly more likely to be H. pylori-positive than women; OR=1.16 (95% CI 1.11–1.22) (12) This was confirmed in a study of adults in Ontario, Canada, in which the overall seroprevalence was 23.1% but higher in men (29.4%) than women (14.9%) (13). One explanation that has been proposed for the lower seroprevalence in women is that they may be more likely to clear H. pylori infection because of higher rates of incidental antibiotic use for other indications (12). There is evidence for a birth cohort effect on H. pylori prevalence; for example, people who were born in the 1930s are more likely to have been infected during childhood than people born in the 1960s. In a study conducted among 7310 US veterans with gastrointestinal symptoms, seroprevalence was 73% among those born before 1920 and 22% in those born after 1980 (14). The overall prevalence of the infection in these US veterans fell from 70.8% in 1997 to a plateau of around 50% after 2002. Within North America, the prevalence of H. pylori infection varies with socioeconomic status and race/ethnicity (14, 15, 16, 17). In general, the prevalence is lower among non-Hispanic whites than among other racial/ethnic groups including African Americans, Hispanic Americans, Native Americans, and Alaska natives (5, 14, 15, 18). African Americans with a higher proportion of African ancestry have been reported to have higher rates of H. pylori infection than African Americans with a lower proportion of African ancestry suggesting that racial/genetic factors may have some role in predisposition to the infection unrelated to socioeconomic factors (16). Higher prevalence rates have been found among those living close to the US/Mexico border (19, 20); in one study (19), prevalence of H. pylori assessed by stool antigen testing was 38.2%. Prevalence has also been reported to be high among Alaska natives (18) and Canadian First Nations populations (21). The prevalence of H. pylori infection is generally lower in the United States than in many other parts of the world, particularly in comparison to Asia and Central and South America (8, 22). There is, however, preliminary evidence that it may be falling in some previously high prevalence areas (22). People immigrating to North America from Asia and other parts of the world have a much higher prevalence of the infection than people born in North America (23). In one study, the seroprevalence among immigrants from East Asia was 70.1% (24). Hispanic immigrants to North America have higher rates of the infection than first- or second-generation Hispanics who were born here (25). Question 2: What Are the Indications to Test For, and to Treat, H. pylori Infection? Recommendations Since all patients with a positive test of active infection with H. pylori should be offered treatment, the critical issue is which patients should be tested for the infection (strong recommendation, quality of evidence: not applicable), All patients with active peptic ulcer disease (PUD), a past history of PUD (unless previous cure of H. pylori infection has been documented), low-grade gastric mucosa-associated lymphoid tissue (MALT) lymphoma, or a history of endoscopic resection of early gastric cancer (EGC) should be tested for H. pylori infection. Those who test positive should be offered treatment for the infection (strong recommendation, quality of evidence: high for active or history of PUD, low for MALT lymphoma, low for history of endoscopic resection of EGC). In patients with uninvestigated dyspepsia who are under the age of 60 years and without alarm features, non-endoscopic testing for H. pylori infection is a consideration. Those who test positive should be offered eradication therapy (conditional recommendation, quality of evidence: high for efficacy, low for the age threshold). When upper endoscopy is undertaken in patients with dyspepsia, gastric biopsies should be taken to evaluate for H. pylori infection. Infected patients should be offered eradication therapy (Strong recommendation, high quality of evidence). Patients with typical symptoms of gastroesophageal reflux disease (GERD) who do not have a history of PUD need not be tested for H. pylori infection. However, for those who are tested and found to be infected, treatment should be offered, acknowledging that effects on GERD symptoms are unpredictable (strong recommendation, high quality of evidence). In patients taking long-term low-dose aspirin, testing for H. pylori infection could be considered to reduce the risk of ulcer bleeding. Those who test positive should be offered eradication therapy (conditional recommendation, moderate quality of evidence). Patients initiating chronic treatment with a non-steroidal anti-inflammatory drug (NSAID) should be tested for H. pylori infection (strong recommendation, moderate quality of evidence). Those who test positive should be offered eradication therapy. The benefits of testing and treating H. pylori in patients already taking NSAIDs remains unclear (conditional recommendation, low quality of evidence). Patients with unexplained iron deficiency (ID) anemia despite an appropriate evaluation should be tested for H. pylori infection. Those who test positive should be offered eradication therapy (conditional recommendation, high quality of evidence). Adults with idiopathic thrombocytopenic purpura (ITP) should be tested for H. pylori infection. Those who test positive should be offered eradication therapy (conditional recommendation, very low quality of evidence). There is insufficient evidence to support routine testing and treating of H. pylori in asymptomatic individuals with a family history of gastric cancer or patients with lymphocytic gastritis, hyperplastic gastric polyps and hyperemesis gravidarum (no recommendation, very low quality of evidence). The ACG’s 2007 treatment guideline on the management of H. pylori infection (26) listed the following as established indications for diagnosis and treatment: Active PUD (gastric or duodenal). Confirmed history of PUD (not previously treated for H. pylori). Gastric MALT lymphoma (low grade). After endoscopic resection of EGC. The current guideline extends the list of potential indications to test patients for H. pylori infection. There are varying levels of evidence in support of the different potential indications for testing that are listed below. For some of these, the decision to test an individual patient for H. pylori will be influenced by clinical judgment and considerations of a patient’s general medical condition. Not all of these potential indications are given a definite recommendation, so that clinicians may exercise their judgment for individual patients. There is no justification in North America for universal or population-based screening. PUD The evidence in support of the 2007 recommendation was substantive at that time and these broad recommendations are still pertinent. All patients with a new diagnosis or a past history of PUD should be tested for H. pylori infection. Ideally, tests which identify active infection such as a urea breath test, fecal antigen test, or when endoscopy is performed, mucosal biopsy-based testing should be utilized. Because of the higher pretest probability of infection, patients with documented PUD represent a rare group, where it is acceptable to utilize an IgG H. pylori antibody test. In most other circumstances where the pretest probability of infection is lower, tests which identify active disease are preferred over antibody testing. Patients with a history of PUD who have previously been treated for H. pylori infection should undergo eradication testing with a urea breath test or fecal antigen test. Patients with evidence of ongoing infection should be treated appropriately. Gastric mucosa-associated lymphoid tissue (MALT) lymphoma The term “MALT lymphoma” has largely been supplanted by “marginal zone B-cell lymphoma of MALT type”. Identification of this neoplasm remains a key indication to test for, and to eradicate, H. pylori infection. A review published in 2009 identified and summarized six prospective cohort studies of treatment for H. pylori infection in patients with gastric MALT lymphoma (also referred to as “localized B-cell lymphoma of the stomach”) but found no systematic reviews or randomized controlled trials (27). Tumor regression was reported in 60–93% of patients after eradication of H. pylori infection, but response was inconsistent, with some patients showing a delayed response and some showing tumor relapse within a year of treatment. More recent studies have confirmed these observations. In a Japanese series, 77% out of 420 patients treated for H. pylori infection showed either complete histological response or probable minimal residual disease (the investigators’ definition of response), although 10 (3%) responders relapsed in a mean of 6.5 years (28). Among infected patients who did not respond to eradication treatment, there was progression of the disease in 27%. Among 120 patients in Germany followed for a median of 122 months, there was initial complete remission in 80% following treatment of H. pylori infection (29). Out of these, 3% had macroscopic recurrence of disease within 24 months, and another 17% had histological residual disease found after a median of 48 months. A recent review has suggested that treatment of H. pylori infection may also be beneficial for patients diagnosed with diffuse large B-cell lymphoma of the stomach (30). Early gastric cancer Three recent meta-analyses have each found that the incidence of metachronous gastric cancer following the endoscopic resection of a gastric neoplasm was reduced by the eradication of H. pylori infection (31, 32, 33). The most inclusive analysis by Yoon et al. (33) included 13 studies (three prospective and 10 retrospective) comprising 6687 patients. The pooled OR of gastric cancer in patients successfully cured of H. pylori was 0.42 (95% CI 0.32–0.56); in a subgroup analysis of the three prospective studies, the OR was 0.39 (95% CI 0.20–0.75) (33, 34). The other two meta-analyses yielded similar results (31, 32). Most recently, a meta-analysis comprising 24 studies (22 out of which were conducted in Asia) confirmed a lower rate of metachronous EGC following treatment of H. pylori infection; the incidence rate ratio was 0.54 (95% CI 0.46–0.65) (34). Dyspepsia (uninvestigated) Dyspepsia (defined as pain or discomfort centered in the upper abdomen) is highly prevalent in North America and elsewhere. In North America, most patients with dyspepsia will not have serious underlying, organic disease to explain their symptoms. That is, most will be found to have functional dyspepsia (FD), which is discussed elsewhere in this guideline. The ACG’s 2007 guideline on H. pylori management (26) included uninvestigated dyspepsia (depending upon H. pylori prevalence) in its list of established indications for diagnosis and treatment of H. pylori infection. The test and treat strategy for H. pylori infection was endorsed for patients under age 55 with dyspeptic symptoms and without alarm features. In the UK, the Bristol Helicobacter Project randomized 1517 H. pylori-positive adults to treatment for H. pylori infection or placebo and followed them prospectively (35). Among those treated for the infection, of whom over 90% achieved successful eradication, there was a small but statistically significant (P<0.05) reduction in subsequent consultations at the primary care level for dyspeptic complaints. The Cochrane Collaboration’s review on initial management strategies for dyspepsia was published in 2005 (36). As of early 2016, it had not been updated. A “test and treat” strategy for H. pylori had been found to be more effective than empirical acid suppression with either a proton pump inhibitor (PPI) or H2-receptor antagonist in managing dyspepsia (relative risk (RR) 0.59; 95% CI 0.42–0.83). This conclusion differs from an individual patient data meta-analysis which included three RCTs of 1537 patients randomized to the “test and treat” strategy or empirical acid suppression for the management of dyspepsia in the primary care setting (37). Although there was no significant difference between the groups in terms of symptom cure at 12 months, there was a trend for reduced overall costs in those assigned to “test and treat”. An individual patient data meta-analysis included five RCTs of 1924 patients randomized to “test and treat” or to prompt upper endoscopy for the evaluation of dyspeptic symptoms (38). After 1 year, the RR of remaining symptomatic was 0.95 (95% CI 0.92–0.99) in favor of prompt endoscopy. However, costs were lower with the “test and treat” approach. Prompt endoscopy for all patients with dyspepsia is neither feasible nor cost-effective. Functional dyspepsia A Cochrane systematic review published in 2006 concluded that there was a small but statistically significant benefit of treating H. pylori infection in patients with FD (39). In 17 RCTs comprising over 3500 patients, the RR reduction seen with treatment of H. pylori infection was 10% (95% CI 6–14%) and the number needed to treat (NNT) to cure one patient with FD was 14 (95% CI 10–25) (39). A subsequent update of that Cochrane review included 21 trials comprising 4331 patients (40). Most trials assessed patients’ symptoms 12 months after treatment. This study validated the NNT of 14 but with a narrower 95% CI (10–20). The Rome IV criteria have suggested subgrouping patients with FD into two groups, epigastric pain syndrome (epigastric pain and/or burning) or post-prandial distress syndrome (meal-related early satiation and/or fullness), while acknowledging that there may be considerable overlap between these (41). Although treatment trials have not utilized these newest criteria, improvement has been shown for patients with either predominant epigastric pain or predominant dysmotility-type symptoms following eradication of H. pylori infection (40). Since some FD patients with H. pylori infection experience durable benefit following eradication therapy, we recommend testing for, and treating, H. pylori in patients with FD. This aligns with a recent guideline by the American Gastroenterological Association, which recommends collecting biopsies of normal-appearing gastric mucosa to test for H. pylori when performing endoscopy in patients with dyspeptic symptoms (42). At the time of preparing this guideline, the ACG and the Canadian Association of Gastroenterology were in the process of preparing a joint guideline on management of uninvestigated dyspepsia and FD (43). Gastroesophageal reflux disease (GERD) There is no proven causal association between H. pylori infection and GERD. On a geographical basis, there is a negative association between the prevalence of H. pylori infection and the prevalence and severity of GERD (44). Barrett’s esophagus is more common among individuals who are not infected with H. pylori (45). The risk of esophageal adenocarcinoma among patients with Barrett’s esophagus is lower among those with H. pylori infection (45). The ACG’s 2007 guideline on H. pylori (26) reviewed the evidence for any change in GERD symptoms or severity following eradication of H. pylori infection. In North Americans who acquire H. pylori infection, the most likely phenotype is antral-predominant gastritis, hypergastrinemia, parietal cell hyperplasia, and increased gastric acid secretion. Such individuals who also have GERD may experience an improvement in GERD symptoms following eradication of H. pylori infection as gastric acid secretion slowly decreases in association with resolution of antral-predominant gastritis and hypergastrinemia (46). In a post hoc analysis of eight RCTs of the treatment of H. pylori infection in duodenal ulcer patients, there was no significant difference in the development of erosive esophagitis or GERD symptoms between those with successful and failed eradication (47). Among patients with pre-existing GERD, there was a worsening of symptoms in 7% of those cured of the infection and in 15% of those with persistent infection (OR=0.47; 95% CI 0.24–0.91; P=0.02). It is theoretically possible, however, that patients with corpus-predominant gastritis may experience onset or worsening of GERD symptoms following H. pylori eradication as a consequence of restitution of parietal cell mass and increased gastric acid secretion. However, this scenario should be relatively uncommon in North America. In a community-based study from the UK (the Bristol Helicobacter Project), treatment for H. pylori infection was not associated with an increase in the prevalence of heartburn or other reflux symptoms (48). Similarly, treatment for H. pylori infection did not improve reflux symptoms in patients with pre-existing symptoms. A systematic review of 27 published studies concluded that eradication of H. pylori infection from patients with duodenal ulcer did not predispose to the development of GERD (49) or worsen symptoms in patients with established GERD (49). Others have reported that cure of H. pylori infection in patients with erosive esophagitis before starting PPI therapy does not influence healing rates or symptom response (50, 51, 52). Therefore, based upon currently available evidence, there is no indication to test a patient with typical GERD symptoms for H. pylori infection unless that patient also has a history of PUD or dyspeptic symptoms. Patients with GERD who are tested for H. pylori infection for any reason and who are found to be positive should be offered treatment for the infection acknowledging that GERD symptoms are unlikely to improve. Long-term treatment with PPIs in H. pylori-positive individuals with corpus-predominant gastritis may promote the development of atrophic gastritis (53, 54). Although eradication of the infection before initiating PPI therapy may prevent the progression to atrophic gastritis (55), the clinical relevance of this is unclear. Low-dose aspirin use Aspirin (acetylsalicylic acid, ASA) is frequently recommended for patients with cardiovascular risk factors or following a major cardiovascular event (56). ASA use increases the risk of upper GI tract ulceration. H. pylori infection is a recognized risk factor for the development of ulcers and for ulcer bleeding during low-dose ASA treatment (57, 58). In a study conducted in Canada, Australia, the UK and Spain, the prevalence of peptic ulcer at endoscopy among 187 middle-aged and elderly patients taking ASA in doses of 75–325 mg daily was 10.7% (95% CI 6.3–15.1%) (59). H. pylori infection was a significant risk factor for ASA-related duodenal ulcer (OR=18.5; 95% CI 2.3–149.4) but not for gastric ulcer (OR=2.3; 95% CI 0.7–7.8). In a study from Hong Kong, patients with an episode of peptic ulcer bleeding while on low-dose ASA were studied according to H. pylori status (60). Those who were cured of H. pylori infection and subsequently re-started on ASA, had a similar rate of recurrent ulcer bleeding as in a previously ASA-naive cohort without bleeding who were started on low-dose ASA. Thus, eradication of H. pylori infection from patients with ASA-associated ulcer bleeding reduces the risk of recurrent bleeding. Regarding other anti-platelet agents, the 2010 expert consensus document jointly prepared by the ACG, the American College of Cardiology Foundation and the American Heart Association acknowledged that H. pylori infection was an established risk factor for upper GI bleeding among patients using thienopyridine anti-platelet agents (61) but made no specific recommendations concerning testing for, or treating, the infection in patients taking these medicines. However, testing for H. pylori will commonly be indicated in patients using these agents since most will also be taking ASA. In the absence of a prospective randomized study addressing H. pylori eradication in North American patients at increased risk for adverse cardiovascular outcomes, we suggest testing for H. pylori when starting prophylactic low-dose aspirin, while acknowledging that the evidence base for this recommendation is weak. Non-steroidal anti-inflammatory drug (NSAID) use H. pylori infection is an independent risk factor for NSAID-induced ulcers and ulcer bleeding (62, 63). Eradication of H. pylori infection before starting NSAID treatment reduces the development of ulcers and risk of ulcer bleeding (64, 65). A 2005 meta-analysis of five RCTs suggested that eradication of H. pylori infection among patients taking NSAIDs was associated with a 57% reduction in the incidence of peptic ulcer (OR=0.43; 95% CI 0.20–0.93) (66). The benefits of H. pylori eradication were greatest in patients who were previously NSAID-naive. Eradication of H. pylori infection before starting NSAID therapy may be the single most cost-effective strategy for the primary prevention of NSAID-associated ulcers in adult patients (67). The benefit of H. pylori eradication in patients already taking NSAIDs is less clear (68). RCTs suggest that H. pylori eradication does not reduce the incidence of new peptic ulcers in chronic NSAID users (69) and that PPI therapy provides a more effective ulcer risk reduction strategy than H. pylori eradication in patients on chronic NSAIDs (66). The ACG’s most recent practice guideline on the prevention of NSAID-related ulcer complications (63) concluded that H. pylori infection increases the risk of NSAID-related GI complications, that there would be at least a potential advantage of testing for the infection in patients requiring long-term NSAID therapy, and that the infection should be eradicated when identified. Iron deficiency anemia H. pylori infection has been associated with ID and iron deficiency anemia (IDA). In a meta-analysis of observational studies, the pooled ORs for ID and IDA in H. pylori-infected individuals were 1.4 (95% CI 1.2–1.6) and 2.0 (95% CI 1.5–2.9), respectively. A separate meta-analysis of 15 observational studies also found that IDA was more prevalent in H. pylori-infected individuals compared with H. pylori-negative controls (OR=2.2; 95% CI 1.5–3.2) (70). Adolescents with IDA have been reported to have a higher prevalence of H. pylori infection than non-anemic controls (71). H. pylori-infected adolescents and adults with IDA respond to oral iron therapy whether or not accompanied by treatment for H. pylori infection. However, the response to iron therapy in H. pylori-infected patients with IDA may be enhanced by the concomitant eradication of the infection (71, 72). A meta-analysis of 16 RCTs conducted among patients with IDA and H. pylori infection found statistically significant differences in favor of H. pylori eradication with oral iron over oral iron alone for increases in hemoglobin (Hgb), serum iron and serum ferritin (SF) levels (P<0.00001 for each) (73). In a separate meta-analysis of four interventional trials, the weighted mean difference in Hgb levels in favor of combined eradication treatment and oral iron vs. oral iron alone was 4.1 g/dl (95% CI −2.6–10.7); that for SF levels in five trials was 9.5 μg/l (95% CI −0.5–19.4) (70). A Cochrane systematic review is being conducted on the topic of the eradication of H. pylori infection for ID but its findings have not yet been reported (74). Idiopathic thrombocytopenic purpura (ITP) There is evidence from small randomized and non-randomized trials for a sustained improvement in platelet counts after eradication of H. pylori infection in a proportion of adult patients with ITP (75, 76, 77). The evidence is less compelling for children with ITP (78). A systematic review of 25 studies (1555 adult patients), all of which included at least 15 patients, found that platelet counts in ITP patients tended to increase after H. pylori eradication (79). Among 696 evaluable patients, 43% achieved a complete response (defined as a platelet count ≥100 × 109/l), and an additional 50% had an overall response (defined as a platelet count ≥30 × 109/l and at least a doubling of the baseline platelet count). Response rates were lower in patients with a baseline platelet count of <30 × 109/l. In general, response rates were higher in regions with high background H. pylori prevalence and in patients with mild degrees of thrombocytopenia. In their practice guideline published in 2011 (80), the American Society of Hematology (ASH) suggested that “screening for H. pylori infection be considered in adults with ITP in whom eradication therapy would be used if testing is positive” (evidence grade 2C). The ASH also recommended that eradication therapy be administered to adults with ITP who were found to have H. pylori based on a test of active infection (evidence grade 1B). The ASH has recommended against testing children with ITP for H. pylori infection (80). Asymptomatic individuals and the risk of gastric cancer Evidence that eradication of H. pylori infection reverses the gastric premalignant changes of gastric atrophy and intestinal metaplasia is conflicting. A meta-analysis of 12 studies (2658 patients) published up until 2009 reported that eradication was associated with a significant reduction in atrophic gastritis in the corpus (P=0.006) but not in the antrum (P=0.06); there was no evidence for a significant effect on intestinal metaplasia in either the corpus (P=0.42) or antrum (P=0.76) (81). A Cochrane systematic review and meta-analysis examined six RCTs (five of which were in Asian populations) that studied the effects of H. pylori eradication treatment against either placebo or no treatment on the subsequent development of gastric cancer among asymptomatic and otherwise healthy-infected adults (82). All trials followed subjects for at least 2 years. The quality of evidence was assessed as moderate. The subsequent incidence of gastric cancer was 1.6% among 3294 treated individuals and 2.4% among 3203 untreated controls (RR=0.66; 95% CI 0.46–0.95). The overall NNT was 124 (95% CI 78–843). However, assuming that the benefits of H. pylori eradication persist for life, the NNT could be as low as 15 among Chinese men. Since the lifetime risk of gastric cancer is lower in the United States, the corresponding NNT to prevent one case here would be 95 for men and 163 for women. A recent meta-analysis of 24 studies, 22 of which were conducted in Asia, showed that treatment of H. pylori infection in asymptomatic, infected adults led to a reduced incidence of gastric cancer. The greatest benefit was seen in people living in regions with the highest incidence of gastric cancer; reported RRs for regions of low, intermediate and high incidence of gastric cancer were, respectively, 0.80, 0.49, and 0.45 (34). Other gastrointestinal and non-gastrointestinal disorders Associations have been proposed between H. pylori infection and numerous other disorders (83). In most cases, biological plausibility and the level of evidence to support a causal association has been weak to non-existent. Thus, no formal recommendation can be offered. Controlled trials have suggested a benefit of H. pylori eradication in healing lymphocytic gastritis (84) and inducing regression of hyperplastic gastric polyps (85). There is some data to suggest a weak association between H. pylori infection and hyperammonemia and hepatic encephalopathy (HE) in patients with cirrhosis (86); trials evaluating H. pylori therapy in patients with HE have yielded conflicting results. In a meta-analysis of observational studies, the prevalence of H. pylori infection in pregnant women with hyperemesis gravidarum was higher than in matched controls (87). An association between H. pylori infection and major cardiovascular events including myocardial infarction and stroke has been postulated. However, evidence is of low quality and insufficient to establish causality (88). A Cochrane systematic review of H. pylori infection in Parkinson’s disease (89) identified three clinical trials and concluded that there was insufficient evidence to support screening for H. pylori infection in this population. Limited evidence suggests that symptoms of Parkinson’s may improve with H. pylori eradication, perhaps due to increases in the absorption and bioavailability of levodopa. Further randomized, controlled trials were encouraged. An evidence-based review of treating H. pylori infection in patients with urticaria found only low-quality evidence (90); nine out of 10 trials identified showed no benefit from H. pylori eradication. A meta-analysis has reported higher levels of glycosylated hemoglobin in H. pylori-positive patients with type 1 diabetes compared with non-infected patients (91). However, glycemic control did not improve in the short term following eradication of H. pylori. An inverse association has been reported between the prevalence of H. pylori infection and obesity (92). There may also be a weak inverse association between H. pylori infection and allergic or atopic disorders (93, 94) including eosinophilic esophagitis (95, 96, 97, 98, 99, 100) as well as celiac disease (101) and inflammatory bowel disease (102). Question 3: What Are Evidence-Based First-Line Treatment Strategies for Providers in North America? Recommendations Patients should be asked about any previous antibiotic exposure(s) and this information should be taken into consideration when choosing an H. pylori treatment regimen (conditional recommendation, moderate quality of evidence). Clarithromycin triple therapy consisting of a PPI, clarithromycin, and amoxicillin or metronidazole for 14 days remains a recommended treatment option in regions where H. pylori clarithromycin resistance is known to be <15% and in patients with no previous history of macrolide exposure for any reason (conditional recommendation, low quality of evidence (for duration: moderate quality of evidence)). Bismuth quadruple therapy consisting of a PPI, bismuth, tetracycline, and a nitroimidazole for 10–14 days is a recommended first-line treatment option. Bismuth quadruple therapy is particularly attractive in patients with any previous macrolide exposure or who are allergic to penicillin (strong recommendation, low quality of evidence). Concomitant therapy consisting of a PPI, clarithromycin, amoxicillin and a nitroimidazole for 10–14 days is a recommended first-line treatment option (Strong recommendation, low quality of evidence (for duration: very low quality of evidence)). Sequential therapy consisting of a PPI and amoxicillin for 5–7 days followed by a PPI, clarithromycin, and a nitroimidazole for 5–7 days is a suggested first-line treatment option (conditional recommendation, low quality of evidence (for duration: very low quality of evidence). Hybrid therapy consisting of a PPI and amoxicillin for 7 days followed by a PPI, amoxicillin, clarithromycin and a nitroimidazole for 7 days is a suggested first-line treatment option (conditional recommendation, low quality of evidence (For duration: very low quality of evidence)). Levofloxacin triple therapy consisting of a PPI, levofloxacin, and amoxicillin for 10–14 days is a suggested first-line treatment option (conditional recommendation, low quality of evidence (for duration: very low quality of evidence)). Fluoroquinolone sequential therapy consisting of a PPI and amoxicillin for 5–7 days followed by a PPI, fluoroquinolone, and nitroimidazole for 5–7 days is a suggested first-line treatment option (conditional recommendation, low quality of evidence (For duration: very low quality of evidence)). H. pylori is an infectious disease that is typically treated with combinations of 2–3 antibiotics along with a PPI, taken concomitantly or sequentially, for periods ranging from 3 to 14 days. In clinical practice, the initial course of eradication therapy, heretofore referred to as “first-line” therapy, generally offers the greatest likelihood of treatment success. Thus, careful attention to the selection of the most appropriate first-line eradication therapy for an individual patient is essential. There is no treatment regimen which guarantees cure of H. pylori infection in 100% of patients. Indeed, there are currently few, if any regimens which consistently achieve eradication rates exceeding 90% (103). In developing this guideline for North America, we conducted comprehensive literature searches to identify randomized, controlled trials which have evaluated the efficacy of treatment regiments in the United States and Canada. Wherever possible, we have tried to highlight these data and use them to develop treatment recommendations. Unfortunately, although H. pylori was the subject of many randomized, controlled trials conducted in North America during the first decade of this century, the number of treatment trials assessing modern regimens is modest to non-existent. As such, we were forced to rely upon clinical trial data generated in other parts of the world when considering a number of regimens. Development of this guideline has made clear the need for clinical trials to evaluate the efficacy of modern treatment regimens and organized efforts to monitor H. pylori antibiotic resistance in North America. To provide readers a sense of the author’s preferences, we have taken the liberty of utilizing the words “recommended” vs. “suggested” in the italicized statements that address each of the treatment regimens. A listing of available first-line treatment options can be found in Table 2. A schema to assist providers to choose the best therapy for an individual patient can be found in Figure 1. Table 2. Recommended first-line therapies for H pylori infection Regimen Drugs (doses) Dosing frequency Duration (days) FDA approval Clarithromycin triple PPI (standard or double dose) BID 14 Yesa Clarithromycin (500 mg) Amoxicillin (1 grm) or Metronidazole (500 mg TID) Bismuth quadruple PPI (standard dose) BID 10–14 Nob Bismuth subcitrate (120–300 mg) or subsalicylate (300 mg) QID Tetracycline (500 mg) QID Metronidazole (250–500 mg) QID (250) TID to QID (500) Concomitant PPI (standard dose) BID 10–14 No Clarithromycin (500 mg) Amoxicillin (1 grm) Nitroimidazole (500 mg)c Sequential PPI (standard dose)+Amoxicillin (1 grm) BID 5–7 No PPI, Clarithromycin (500 mg)+Nitroimidazole (500 mg)c BID 5–7 Hybrid PPI (standard dose)+Amox (1 grm) BID 7 No PPI, Amox, Clarithromycin (500 mg), Nitroimidazole (500 mg)c BID 7 Levofloxacin triple PPI (standard dose) BID 10–14 No Levofloxacin (500 mg) QD Amox (1 grm) BID Levofloxacin sequential PPI (standard or double dose)+Amox (1 grm) BID 5–7 No PPI, Amox, Levofloxacin (500 mg QD), Nitroimidazole (500 mg)c BID 5–7 LOAD Levofloxacin (250 mg) QD 7–10 No PPI (double dose) QD Nitazoxanide (500 mg) BID Doxycycline (100 mg) QD BID, twice daily; FDA, Food and Drug Administration; PPI, proton pump inhibitor; TID, three times daily; QD, once daily; QID, four times daily. a Several PPI, clarithromycin, and amoxicillin combinations have achieved FDA approval. PPI, clarithromycin and metronidazole is not an FDA-approved treatment regimen. b PPI, bismuth, tetracycline, and metronidazole prescribed separately is not an FDA-approved treatment regimen. However, Pylera, a combination product containing bismuth subcitrate, tetracycline, and metronidazole combined with a PPI for 10 days is an FDA-approved treatment regimen. c Metronidazole or tinidazole. Figure 1. Selection of a first-line H. pylori treatment regimen. With very few exceptions, the most common adverse events associated with antibiotics used to treat H. pylori infection are gastrointestinal in origin (for example: nausea, dysgeusia, dyspepsia/abdominal pain, diarrhea). As such, we have not listed adverse events for most of the therapies. Where unusual adverse events can occur with a specific therapy, we have tried to point that out. Clarithromycin triple therapy The previous ACG guideline from 2007 recommended 14 days of treatment with a PPI, clarithromycin, and amoxicillin (clarithromycin-based triple therapy) or—in patients with an allergy to penicillin—metronidazole as an alternative to amoxicillin. At that time, eradication rates for clarithromycin triple therapy were reported to be 70–85% and were highly influenced by the underlying rate of clarithromycin resistance (26). However, there has been growing concern regarding the efficacy of clarithromycin triple therapy. Key questions which were considered while preparing this document included the expected eradication rate of clarithromycin triple therapy in North America, the most appropriate duration of therapy, and whether eradication rates have been dropping over time. There are data from other parts of the world to suggest that eradication rates for clarithromycin triple therapy are below 80% (103). In preparing this updated guideline, we identified all randomized, controlled trials conducted in the United States or Canada which have assessed the efficacy of this regimen since 2000 (100, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119). Consistent with other meta-analyses, eradication rates with 7 or 10 days of clarithromycin triple therapy in studies from the US or Canada were indeed below 80%. Eradication rates with 14 days of triple therapy were higher, but only two study arms with 195 subjects were included. This finding is consistent with the most recent and most complete meta-analysis or the world’s literature on this topic which was published by the Cochrane Collaboration (120). For clarithromycin triple therapy, higher eradication rates were reported with 14 vs. 7 days of treatment (34 studies, RR 0.65, 95% CI 0.57–0.75; NNT 12, 95% CI 9–16), and with 14 vs. 10 days (10 studies, RR 0.69, 95% CI 0.52–0.91). Based upon the available data, when triple therapy is utilized in North America, it should be given for 14 days. The lack of recent RCT data on clarithromycin triple therapy makes it difficult to confidently report on temporal trends in eradication rates. To address this issue, we conducted a retrospective analysis of eradication rates for clarithromycin triple therapy at the University of Michigan from 2001–15. Data was divided into 5-year blocks and eradication rates for 10–14 days of triple therapy when given first-line were calculated. In 662 patients, the overall eradication rate was 79.5% (95% CI 77.2–82.4%) with no significant difference in eradication rates for the 3, 5-year blocks (Figure 2, unpublished data). Figure 2. Eradication rates with first-line clarithromycin triple therapy at the University of Michigan (2001–2015). The impact of clarithromycin resistance on the efficacy of clarithromycin triple therapy is well documented. A 2010 meta-analysis reported an eradication rate of 22% for clarithromycin-resistant H. pylori strains compared with 90% for clarithromycin-sensitive strains (121). As such, clarithromycin triple therapy should not be utilized in areas where the rate of clarithromycin resistance is known to be high. The Maastricht/Florence Consensus document published in 2012 recommended against using triple therapy when the rate of underlying clarithromycin resistance exceeds 15–20% (55). Although current large scale data on H. pylori antibiotic resistance in North America are unavailable, recent data from Houston suggest that clarithromycin resistance rates may now fall within that range (122). In the absence of local or even regional H. pylori antibiotic resistance data, it is very important to ask patients about previous exposure to antibiotics for any reason, particularly macrolides and fluoroquinolones, as this provides a proxy for underlying H. pylori antibiotic resistance (123, 124). A recent study confirmed an association between number of previous antibiotic exposures and an increasing risk for antibiotic resistance (125). Similarly, duration of previous macrolide therapy for greater than 2 weeks is also associated with a greater risk of treatment failure with clarithromycin triple therapy (126). Based upon the available data, we conclude that clarithromycin triple therapy consisting of a PPI, clarithromycin, and amoxicillin or metronidazole for 14 days remains a first-line treatment option in regions where H. pylori clarithromycin resistance is known to be low. In regions where clarithromycin resistance exceeds 15%, as may well be the case in many parts of North America, clarithromycin triple therapy should be avoided. All patients should be asked about previous macrolide exposure for any reason. In those with previous macrolide exposure, clarithromycin triple therapy should be avoided. Bismuth quadruple therapy The previous ACG guideline also endorsed the use of 10–14 days of bismuth quadruple therapy composed of a PPI or histamine-2 receptor antagonist, bismuth, metronidazole, and tetracycline. There is very limited data on the efficacy or comparative effectiveness of bismuth quadruple therapy in North America. A literature search identified only two RCTs which included a bismuth quadruple therapy arm (n=172). The mean eradication rate with this regimen given for 10 days was 91% (95% CI; 81–98%). A meta-analysis of studies from around the world comparing clarithromycin triple and bismuth quadruple therapies suggested that the two treatments had similar efficacy, compliance, and tolerability (121). An updated meta-analysis, which included 12 RCTs and 2753 patients, reported intention-to-treat (ITT) eradication rates of 77.6% with bismuth quadruple therapy vs. 68.9% with clarithromycin triple therapy (risk difference=0.06, 95% CI; −0.01 to 0.13). There was significant heterogeneity in the data set, in part attributable to differences in trea

|
Scooped by
Gilbert C FAURE
August 22, 2017 4:32 AM
|
The use of antibiotic therapy early in life might influence the risk of developing asthma. Studies assessing the influence of early life antibiotic use on the risk of asthma exacerbations ar

|
Scooped by
Gilbert C FAURE
February 7, 2017 4:59 AM
|
Many people who think they're allergic to penicillin don't really have an allergy to this antibiotic, a pediatric expert says.

|
Scooped by
Gilbert C FAURE
November 13, 2014 1:33 AM
|
Allergy Immunology | ATLANTA — Ninety-four percent of patients who reported a history of penicillin allergy and were scheduled to undergo surgery had negative penicillin allergy skin test results, according to data presented here.

|
Scooped by
Gilbert C FAURE
April 29, 2014 8:43 AM
|
PubMed comprises more than 23 million citations for biomedical literature from MEDLINE, life science journals, and online books. Citations may include links to full-text content from PubMed Central and publisher web sites.

|
Scooped by
Gilbert C FAURE
January 23, 2014 3:03 PM
|

|
Scooped by
Gilbert C FAURE
September 2, 2013 6:29 AM
|
Antibiotic Hypersensitivity and Treatment Changes
Infectious Disease Special Edition
David J.

|
Scooped by
Gilbert C FAURE
November 9, 2012 2:31 PM
|
Eight weeks after antibiotic treatment of infants, the diversity of gastrointestinal flora remained diminished, although the number of individual bacteria was back to normal, according to a new paper.
|


 Your new post is loading...
Your new post is loading...
 Your new post is loading...
Your new post is loading...