 Your new post is loading...
 Your new post is loading...

|
Suggested by
Société Francaise d'Immunologie
October 26, 2018 4:00 PM
|
Original Article from The New England Journal of Medicine — Efficacy and Safety of Dupilumab in Glucocorticoid-Dependent Severe Asthma...

|
Scooped by
Gilbert C FAURE
October 25, 2018 1:34 PM
|
Hereditary angioedema due to C1 inhibitor deficiency (C1-INH-HAE) is a rare autosomal dominant disease characterized by episodes of acute subcutaneous swelling, and/or recurrent severe abdominal pain. The disease is potentially fatal if the upper-airway is involved. Iatrogenic harm can occur if HAE is not considered in the differential diagnosis, the specialists are not aware of the natural history, diagnosis and treatment of HAE, or as a result of unnecessary surgical and other iatrogenic interventions. We present the case of a 72-year-old man who began suffering recurrent abdominal pain at the age of 8 years. The pain led to frequent emergency department visits, three emergency surgical interventions, and 5 endoscopies before C1-INH-HAE was diagnosed at the age of 70. Infrequent subcutaneous swellings were attributed to unknown allergic reactions that were not related to the primary diagnosis of abdominal pain. Family history was positive for recurrent abdominal pain and angioedema but was ignored until the propositus’ grandson developed recurrent severe oro-facial edema attacks. The boy’s mother searched the worldwide web and found educational materials on a patient association website. She suggested complement C4 and C1-INH testing that led to the appropriate diagnosis of C1-INH-HAE type 1 in her son and his grandfather. This report emphasizes the importance of accurately evaluating personal and family history in patients with a long history of recurrent, acute, severe but medically unexplained abdominal pain and cutaneous swellings. Here, the diagnosis of HAE was overlooked for 62 years and the focus on abdominal complaints led to numerous surgical interventions without consideration of the full differential diagnosis. Screening family members from all generations for unrecognized angioedema, abdominal pain, and measurement of C1-INH and C4 are essential for accurate and timely diagnosis of HAE.

|
Scooped by
Gilbert C FAURE
October 16, 2018 1:52 AM
|
Allergen-specific immunotherapy (ASIT) has the potential to modify allergic diseases, and it is also considered a potential therapy for allergic asthma. House dust mite (HDM) allergens, a common source of airborne allergen in human diseases, have been developed as an immunotherapy for patients with allergic asthma via the subcutaneous and sublingual routes. Oral immunotherapy with repeated allergen ingestion is emerging as another potential modality of ASIT. The aim of this study was to evaluate the therapeutic efficacy of the oral ingestion of HDM extracts in a murine model of allergic asthma. BABL/c mice were sensitized twice by intraperitoneal injection of HDM extracts and Al(OH)3 on day 1 and day 8. Then, the mice received challenge to induce airway inflammation by intratracheal instillation of HDM extracts on days 29–31. The treatment group received immunotherapy with oral HDM extracts ingestion before the challenge. All the mice were sacrificed on day 32 for bronchoalveolar inflammatory cytokines, mediastinal lymph node T cells, lung histology, and serum HDM-specific immunoglobulins analyses. Upon HDM sensitization and following challenge, a robust Th2 cell response and eosinophilic airway inflammation were observed in mice of the positive control group. The mice treated with HDM extracts ingestion had decreased eosinophilic airway inflammation, suppressed HDM-specific Th2 cell responses in the mediastinal lymph nodes, and attenuated serum HDM-specific IgE levels. Oral immunotherapy with HDM extracts ingestion was demonstrated to have a partial therapeutic effect in the murine model of allergic asthma. This study may serve as the basis for the further development of oral immunotherapy with HDM extracts in allergic asthma.

|
Scooped by
Gilbert C FAURE
October 15, 2018 1:11 PM
|
‘Asthma’ is now recognised as an umbrella term that includes a heterogeneous group of phenotypes whose aetiology and prognosis vary. This heterogeneity is even more significant in asthma diagnosed before 5 years, using the symptom of wheeze as the predominant criterion, even though not all wheeze...

|
Scooped by
Gilbert C FAURE
October 6, 2018 5:19 AM
|

|
Scooped by
Gilbert C FAURE
October 4, 2018 8:14 AM
|
Back to listingNews Scientists develop a new test to safely and accurately diagnose peanut allergies 3 May 2018 MRC scientists have developed a new laboratory test to diagnose peanut allergy. The test has 98% specificity and, unlike current options, it doesn’t run the risk of false-positives or causing allergic reactions such as anaphylactic shock. The simple blood test is five times more cost-efficient compared to the oral food challenge (OFC) – the standard food allergy test – and could be adapted to test for other food allergies. Peanut allergies are among the most common food allergies in children*. Currently, doctors diagnose peanut allergy using a skin-prick test or specific IgE test but this may result in over-diagnosis or false-positives and it cannot differentiate between sensitivity and true food allergy. When skin-prick and IgE test results are unclear, allergists rely on an OFC, which consists of feeding peanut in incrementally larger doses to a patient in a highly-controlled setting in hospital to confirm allergy to the food. While the test is the gold-standard for diagnosing food allergies, there is risk of causing severe allergic reactions. Now, the researchers have developed a safer, accurate blood test in the lab. The new test, called the mast activation test (MAT), could act as a second line tool when skin-prick test results are inconclusive and before referring children and their families to specialists for an OFC, according to researchers from the MRC & Asthma UK Centre in Allergic Mechanisms of Asthma. Their new study was published in the Journal of Allergy and Clinical Immunology. Dr Alexandra Santos, an MRC Clinician Scientist at King’s College London, paediatric allergist and study lead author, said: “The current tests are not ideal. If we relied on them alone, we’d be over diagnosing food allergies – only 22% of school-aged children in the UK with a positive test to peanuts are actually allergic when they’re fed the food in a monitored setting.” Dr Santos continued: “The new test is specific in confirming the diagnosis so when it’s positive, we can be very sure it means allergy. We would reduce by two-thirds the number of expensive, stressful oral food challenges conducted, as well as saving children from experiencing allergic reactions.” Food allergy symptoms are triggered when allergens interact with an antibody called immunoglobulin E (or IgE). The food allergens activate IgE antibodies, triggering symptoms such as skin reactions, itching or constricting of the mouth, throat and airways, and digestive problems (such as stomach cramps, nausea or vomiting). The current skin-prick test and IgE test, which have been in use for decades, measure the presence of IgE antibodies. The new test focuses on mast cells, which play a crucial role in triggering allergic reactions. Mast cells activate by recognising the IgE in plasma and, in allergic patients, produce biomarkers associated with allergic reactions, which can be detected in the lab. Using blood samples from 174 children participating in allergy testing – 73 peanut allergic and 101 peanut-tolerant – the scientists added peanut protein to mast cells to screen for IgE-mediated activation. The MAT accurately identified peanut allergy with 98 specificity. (Specificity is a statistical measure in determining efficacy for diagnosis. The MAT test rarely gives positive results in non-allergic patients.) The researchers also found the test reflected the severity of peanut allergy – patients with more severe reactions have a higher number of activated mast cells. The MAT test is five times cheaper to conduct than the OFC, which requires an allergist and specialist nurses on hand to monitor for adverse reactions and provide medical support if symptoms arise. Dr Santos said: “We are adapting this test to other foods, such as milk, eggs, sesame and tree nuts. This test will be useful as we are seeing more and more children who have never been exposed to these foods because they have severe eczema or have siblings with allergies. Parents are often afraid to feed them a food that is known to cause allergic reactions.” The researchers believe the MAT test may have other uses, for example, in the food industry to detect the presence of allergens in products. Pharmaceutical companies could use it to monitor patients’ allergic response to drugs being evaluated during clinical trials. The scientists plan to transition the biomarker test out of the laboratory and into a clinical setting. They will be testing blood samples from patients with suspected allergies to further validate its utility. 5 to 8% of UK children have a food allergy with up to one in 55 children having a peanut allergy, according to Food Standards Agency estimates. Current UK guidelines recommend avoiding giving your child peanuts and foods containing peanuts before the age of six months. Other countries, such as Canada and the United States, have updated their recommendations – a move that is in the works in the UK. The researchers say updated guidelines may result in a rise in requests for peanut allergy diagnosing. This paper is available on Europe PMC. Categories Categories: Research Health categories: Blood, Inflammatory, Respiratory, Generic Strategic objectives: Lifestyles affecting health, Environment and health, Securing impact from medical research, Aim: Picking research that delivers, Aim: Research to people, Aim: Supporting scientists Locations: London Type: News article

|
Scooped by
Gilbert C FAURE
September 20, 2018 9:20 AM
|
![Figure][1]
Th2 immune response is critical for allergic asthma pathogenesis. Molecular mechanisms for regulating Th2 immunity are still not well understood. Here we report that the ubiquitin-specific protease USP38 is crucial for Th2-mediated allergic asthma. TCR stimulation up-regulated the USP38 level, and USP38 in turn mediated the protein stabilization of JunB, a transcription factor specific for Th2 development. Consequently, USP38 was specifically required for TCR-induced production of Th2 cytokines and Th2 development both in vitro and in vivo, and USP38-deficient mice were resistant to asthma pathogenesis induced by OVA or HDM. Mechanistically, USP38 directly associated with JunB, deubiquitinated Lys-48–linked poly-ubiquitination of JunB, and consequently blocked TCR-induced JunB turnover. USP38 represents the first identified deubiquitinase specifically for Th2 immunity and the associated asthma.
[1]: pending:yes

|
Scooped by
Gilbert C FAURE
September 10, 2018 1:22 PM
|
Nucala demonstrated greater reduction in exacerbations and improved asthma control.

|
Scooped by
Gilbert C FAURE
September 3, 2018 8:09 AM
|
Thinking of traveling by plane or train? Or are you taking a road trip? Plan ahead so that asthma and allergies do not flare up during a special adventure.

|
Scooped by
Gilbert C FAURE
August 10, 2018 2:30 PM
|
Diversity of T cells & asthma subtypes.

|
Scooped by
Gilbert C FAURE
August 6, 2018 6:21 AM
|
In addition to DNA release, neutrophil extracellular trap (NET) formation can result in enucleated cells called cytoplasts. Krishnamoorthy et al . examined how neutrophil cytoplasts contribute to asthmatic inflammation in mouse models of allergic lung inflammation and in asthmatic patients. Airway exposure of mice to LPS with house dust mite allergen induced NET formation in the lung that was associated with IL-17 production upon subsequent exposure to allergen. Cytoplasts and not neutrophil DNA released in NETosis triggered neutrophilia upon allergen exposure, and cytoplasts alone were sufficient to induce IL-17 production by antigen-specific T cells. Cytoplasts also correlated with IL-17 levels in bronchoalveolar lavage fluid from severe asthmatics. These findings provide insight into how neutrophil cytoplasts can contribute to asthma severity.
Severe asthma is a debilitating and treatment refractory disease. As many as half of these patients have complex neutrophil-predominant lung inflammation that is distinct from milder asthma with type 2 eosinophilic inflammation. New insights into severe asthma pathogenesis are needed. Concomitant exposure of mice to an aeroallergen and endotoxin during sensitization resulted in complex neutrophilic immune responses to allergen alone during later airway challenge. Unlike allergen alone, sensitization with allergen and endotoxin led to NETosis. In addition to neutrophil extracellular traps (NETs), enucleated neutrophil cytoplasts were evident in the lungs. Surprisingly, allergen-driven airway neutrophilia was decreased in peptidyl arginine deiminase 4–deficient mice with defective NETosis but not by deoxyribonuclease treatment, implicating the cytoplasts for the non–type 2 immune responses to allergen. Neutrophil cytoplasts were also present in mediastinal lymph nodes, and the cytoplasts activated lung dendritic cells in vitro to trigger antigen-specific interleukin-17 (IL-17) production from naïve CD4+ T cells. Bronchoalveolar lavage fluid from patients with severe asthma and high neutrophil counts had detectable NETs and cytoplasts that were positively correlated with IL-17 levels. Together, these translational findings have identified neutrophil cytoplast formation in asthmatic lung inflammation and linked the cytoplasts to T helper 17–mediated neutrophilic inflammation in severe asthma.

|
Scooped by
Gilbert C FAURE
July 30, 2018 6:27 AM
|
The growing prevalence of allergy and asthma in India has become a major health concern with symptoms ranging from mild rhinitis to severe asthma and even life-threatening anaphylaxis.The “allergen r...

|
Scooped by
Gilbert C FAURE
July 23, 2018 2:27 PM
|
![Figure][1]
Allergic asthma is a chronic inflammatory disease primarily mediated by Th2 immune mechanisms. Numerous studies have suggested that early life exposure to lipopolysaccharide (LPS) is negatively associated with allergic asthma. One proposed mechanism invokes desensitization of lung epithelial cells by LPS. We report here that acyloxyacyl hydrolase (AOAH), a host lipase that degrades and inactivates LPS, renders mice more susceptible to house dust mite (HDM)–induced allergic asthma. Lung epithelial cells from Aoah−/− mice are refractory to HDM stimulation, decreasing dendritic cell activation and Th2 responses. Antibiotic treatment that diminished commensal LPS-producing bacteria normalized Aoah−/− responses to HDM, while giving LPS intrarectally ameliorated asthma. Aoah−/− mouse feces, plasma, and lungs contained more bioactive LPS than did those of Aoah+/+ mice. By inactivating commensal LPS, AOAH thus prevents desensitization of lung epithelial cells. An enzyme that prevents severe lung inflammation/injury in Gram-negative bacterial pneumonia has the seemingly paradoxical effect of predisposing to a Th2-mediated airway disease.
[1]: pending:yes
|

|
Rescooped by
Gilbert C FAURE
from Immunology and Biotherapies
October 26, 2018 1:56 AM
|
Allergen immunotherapy (AIT) is a safe, effective treatment for allergic rhinoconjunctivitis and allergic asthma. However, AIT's clinical effect is still contested—primarily due to heterogeneity in clinical trial designs, study populations, therapeutic formulations, and efficacy criteria. After discussing current concepts and unmet needs, an international panel of experts made several recommendations: (i) explore and validate definitions for (clinical) responders in AIT trials; (ii) use of well‐documented, standardized provocation tests prior to inclusion of subjects with relevant diseases in AIT trials; (iii) monitoring neo‐sensitizations and occurrence of new allergy in extended AIT trials, and exclusion of polyallergic participants; (iv) validation of allergen exposure chambers with regard to natural exposure; (v) in studies of seasonal allergies, focus on peak exposure but also consider organizing two parallel, geographically distinct but otherwise identical trials; (vi) discuss adaptive trial designs with the regulatory authorities; (vii) use e‐health and m‐health technologies to capture more information on individual exposure to allergens; (viii) initiate research on potential psychological, biochemical, immune, neural, and even genomic markers of the placebo response; (ix) identify trial designs and primary endpoints that will give children with allergies easier, faster access to AIT formulations; and (x) promote and apply standardized methods for reporting systemic and local adverse events. The latest technologies and trial designs may provide novel, ethical ways of reducing bias and heterogeneity in AIT clinical trials. There is scope for physicians, patient organizations, companies, and regulators to improve clinical trials in AIT and, ultimately, to provide patients with better treatments.
Via Krishan Maggon

|
Rescooped by
Gilbert C FAURE
from Immunology and Biotherapies
October 23, 2018 2:56 AM
|
Ingesting small doses of peanut products guards against allergic reactions—but an undercurrent of anxiety persists.
![Figure][1]
Jacob Kingsley, 12, visits a bakery that was off-limits before he began oral immunotherapy for a peanut allergy.
PHOTO: MADDIE MCGARVEY
Jacob Kingsley was 9 years old when he was handed the poison he'd shunned since before he could walk and told to swallow it as medicine. Obediently, he gulped down a few micrograms of peanut flour—less than 1/1000 of a peanut—diluted in grape Kool-Aid. His mother and a nurse hovered, ready to inject him with epinephrine if an itchy throat and wheezing struck.
Jacob's mother, Jennifer Kingsley, had driven him 2 hours from their home in Columbus to this doctor's office in Cincinnati, Ohio, for the first of dozens of sessions of peanut immunotherapy. Giving Jacob gradually increasing doses of peanuts, she hoped, would desensitize his immune system.
It's a strategy Kingsley hadn't pursued until she reached her breaking point. A year earlier, Jacob had swallowed a handful of popcorn that, unbeknownst to him, was laced with peanut product. He suffered a particularly frightening reaction: two bouts of intense symptoms about 6 hours apart. The incident marked his second peanut-related trip to the emergency room, and Kingsley was terrified that the next encounter could be fatal. “I decided, ‘I can't live like this,’” she says. “I was desperate.”
As Jacob sat through the hourslong appointment in Cincinnati, playing video games and swigging increasing doses of peanut-spiked Kool-Aid, he joined legions of children writing food allergy's next chapter. Today, more than 3000 people worldwide, most of them children, have undergone peanut immunotherapy, with the goal of protecting them if they accidentally encounter the food. Other children are trying immunotherapy for allergies to milk, eggs, and tree nuts. Some, like Jacob, get treatment in allergists' offices, where doctors share protocols informally and in published papers. Other children have enrolled in clinical trials, including those run by two companies racing to introduce a peanut-based capsule or skin patch. Both plan to apply for approval from the Food and Drug Administration (FDA) this year. The agency's blessing would dramatically boost immunotherapy's credibility and reach.
In a field that for decades has had nothing to offer patients beyond avoidance, immunotherapy marks a seismic shift. As it edges closer to mainstream, “There's mixed feelings, with a whole range of enthusiasm,” says Corinne Keet, a pediatric allergist-immunologist at Johns Hopkins Medicine in Baltimore, Maryland. Fear that it might cause harm is mingling with euphoria that children living constrained lives could be set free. Doctors who offer immunotherapy describe families eating in Chinese restaurants for the first time and home-schooled children rejoining their peers.
Like many medical firsts, the therapy is not perfect. “This is version 1.0,” says Brian Vickery, a pediatric allergist-immunologist at Emory University in Atlanta. He has conducted peanut immunotherapy trials and worked for 2 years at Aimmune Therapeutics, headquartered in Brisbane, California, one of the companies whose products are nearing approval. Physicians fret about oral immunotherapy's rigors—treatment must continue indefinitely—and its risks, which include the same allergic reactions it aims to prevent. Last year in Japan, a child suffered brain damage during a trial of immunotherapy for milk allergies.
Meanwhile, physicians on the front lines are navigating hazy science. No one knows exactly how immunotherapy works or who's most likely to be helped or hurt by it. “For me,” Keet says, “it's really not clear for an average child with peanut allergy whether it will make sense to do oral immunotherapy or not.”
LIKE MANY WHO STUDY food allergies, Keet was enticed by their mystery. Animal models are poor. The intensity of allergic reactions varies unpredictably, even in the same person over time. Why one child outgrows an allergy and another doesn't is unknown.
“This was something we didn't cover much in medical school” in the 1990s, says Matthew Greenhawt, a pediatric allergist-immunologist at Children's Hospital Colorado in Denver. Greenhawt's career trajectory tracks with a surge in food allergies, and these days, he can barely keep up with the stream of affected children who visit his hospital. Today, between 1% and 2% of people in the United States, the United Kingdom, and several other countries are allergic to peanuts—a rate that has roughly tripled since the mid-1990s. Other food allergies, such as those to tree nuts, are also on the rise. What's causing the increase is not well understood.
Despite rising caseloads, deaths from food allergies remain rare. Precise numbers are hard to come by, and estimates range from fewer than 10 to more than 150 a year in the United States. But even though an affected child is more likely to be struck by lightning than to die of a food allergy, the risk can feel ever-present. Parents never know when their children will happen upon culprit foods and how they'll be affected if they do. “We live in a complex world—people move food all over the place,” says David Bunning, a businessman whose two sons, now adults, have multiple food allergies. “The impact on children in terms of their confidence to explore their environment can be extreme.” Bunning's family almost never traveled or ate out. At their grandparents' house, the boys were usually confined to one room where food wasn't allowed.
Bunning now chairs the board of directors at Food Allergy Research & Education (FARE), an advocacy group in McLean, Virginia. Families like his, and the doctors who cared for their children, began to agitate for new treatments about a decade ago. Immunotherapy was the obvious candidate: Injections that desensitize the immune system to pollen, grass, pet dander, and bee venom have been around for decades.
Whether for an allergy to cats or pistachios, immunotherapy aims to disrupt the cells that swing out of control when faced with an allergen. When a child who is allergic to a food eats it, food proteins cross from the digestive tract into the bloodstream. An antibody called immunoglobulin E (IgE), which is bound to white blood cells called mast cells in tissues, recognizes the culprits. IgE activates the mast cells, which release histamine and other chemicals. In the skin, that response can lead to hives; in the respiratory tract, wheezing; and in the gut, vomiting. The most serious symptoms, such as a swollen throat or a reaction throughout the body, mark anaphylaxis, which is what families fear the most. Allergy shots blunt production of IgE, in part, researchers believe, by boosting levels of certain T cells that prompt a cascade of immune changes.
![Figure][1]
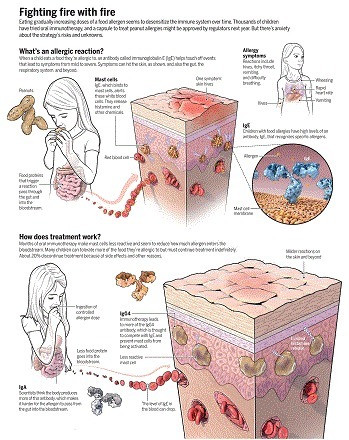
Fighting fire with fire
Eating gradually increasing doses of a food allergen seems to desensitize the immune system over time. Thousands of children have tried oral immunotherapy, and a capsule to treat peanut allergies might be approved by regulators next year. But there's anxiety about the strategy's risks and unknowns.
GRAPHIC: C. BICKEL/ SCIENCE
Brief testing decades ago indicated that shots for food allergies weren't safe. So around the mid-2000s, scientists began to feed children the allergen instead. One watershed moment came in 2005, when the National Institutes of Health formed a consortium for food allergy clinical trials. A second was in 2011, when advocates sponsored a symposium at Harvard Medical School in Boston to standardize goals and strategy for the pioneering immunotherapy efforts. About 60 people attended. “The patients were very clear,” says Carla McGuire Davis, a pediatric allergist-immunologist at Texas Children's Hospital in Houston. They didn't care about eating a peanut butter sandwich; they wanted protection if they accidentally encountered one. Trialists set their end dose at a couple of peanuts and pressed ahead.
The results of early clinical trials were promising, says Hugh Sampson, a pediatric allergist-immunologist at the Icahn School of Medicine at Mount Sinai in New York City, who has studied immunotherapy in food allergies for many years. After 6 to 12 months of treatment, he says, about 70% to 80% of patients could handle higher doses of the food than before. Lab data were encouraging, too: Ingesting allergens over time seems to make mast cells less reactive, inhibiting their release of harmful chemicals. The therapy also produces other immunoglobulins: IgG4, which further inhibits mast cell activity, and IgA, which helps keep food allergens from escaping the gut (see graphic, p. 280).
The 2011 conference inspired the founding of the company now called Aimmune, fueled by more than $3.5 million from FARE. A second company, DBV Technologies, based in Montrouge, France, and New York City, expanded a few years later. Aimmune began to develop an oral product, essentially a capsule of powder derived from peanut flour with proteins held to consistent levels. In February, the company announced in a press release the results of a phase III trial involving 496 children and teenagers, with a regimen stepping up every 2 weeks through 11 dose levels. Among the 372 people in the treatment group, about 20% dropped out for various reasons, including side effects. After about a year, 96% of people who completed treatment could consume one peanut with no more than mild symptoms, 84% could tolerate two, and 63% could tolerate at least three.
DBV's skin patch represents a more conservative strategy: It delivers tiny amounts of peanut protein, the equivalent of one peanut over 3 years. Last year, DBV announced that in its phase III trial of almost 400 patients, after a year, those using the patch could, on average, eat three peanuts over the course of several hours before experiencing clinical symptoms such as vomiting or hives; before the trial, the average was just under one peanut. Outcomes varied substantially from person to person.
If one or both products are approved by FDA in the coming months, expectations are high that they'll be welcomed: Aimmune is now worth about $1.5 billion on the U.S. stock exchange. In 2016, FARE sold its share in Aimmune for $47 million.
MEANWHILE, SOME DOCTORS embrace another route: offering peanut immunotherapy in their practices. “I can treat 20 patients with $5.95 of peanut flour,” says Richard L. Wasserman, a pediatric allergist-immunologist in Dallas, Texas.
Wasserman ventured into food allergy immunotherapy 11 years ago. He developed a protocol based partly on published case reports and protocols for allergy shots, and he put IVs into his first five peanut allergy patients in case he had only seconds to rescue them from severe anaphylaxis. “When they all sailed through the first day, we stopped doing IVs,” he says. “But that's a measure of how concerned I was.”
Wasserman has since treated more than 300 children with peanut allergies and more than 400 with other food allergies. Other practitioners are joining in, among them the Cincinnati allergist whom the Kingsley family sought out: Justin Greiwe at Bernstein Allergy Group. Greiwe joined the practice in 2014, straight out of medical training. “It was a little nerve-wracking at the beginning,” he says, because no officially sanctioned oral immunotherapy protocol existed. He took precautionary measures, such as lung testing before every treatment, to help ensure patient safety.
Some clinicians—and executives at the companies developing products—aren't happy about the doctor's office treatments. “That gives a lot of us pause,” says Sampson, who in addition to his academic post is chief scientific officer of DBV. “We're very afraid that if this goes on enough, somebody is going to have an accident or a fatal reaction, and that's really going to change the FDA's viewpoint” about the products in development, he says.
Wasserman agrees about the need for caution. “Not every practicing allergist should be doing oral immunotherapy,” he says. Greiwe suggests the treatment requires a dedicated staff, and he gives every immunotherapy family his cellphone number.
Jacob was one of Greiwe's first immunotherapy patients. His mother remembers Jacob's ears burning—a minor reaction that subsided on its own. “Or he said he hated peanuts and wanted to quit,” she says. Worst was about 6 months in, when Kingsley discovered that for 2 weeks, Jacob had hidden his dose to avoid eating it. That was “the only time we ever felt danger,” she says. Stopping treatment can quickly alter the immune system, says Cecilia Berin, an immunologist at Mount Sinai, because immunotherapy requires constant exposure. When Jacob squirreled away his daily dose, the changes induced in his immune system almost certainly started to fade out, putting him at risk. Greiwe restarted him on a lower dose and, his mother says, “We got through it.”
EVEN CHILDREN WHO faithfully follow instructions face risks. The immune system can react to even subtle pressures, and the list of what can provoke a reaction to treatment is long. Exercising within a couple of hours of the dose can do it; so can a cold, a stomach virus, menstruation, or a hot shower. An asthma attack can trigger a reaction—many children with allergies have asthma as well—and so can stress. “We had a patient who had just played the violin on a stage, came down, and about 15 minutes later … took the dose and had a reaction,” Davis says.
Berin posits that external pressures such as physical activity or illness make the gut more permeable, pushing more of the immunotherapy dose into the bloodstream. But that remains hypothesis. Regardless, it's becoming clear that “there are people who react years down the road to a maintenance dose,” Keet says. For Jacob, such a moment came 9 months in. One evening while watching a movie, he downed his peanut M&M's and later ran outside with his cousins to dance in a rainstorm. He broke out in hives head to toe. Kingsley dialed Greiwe's number, and Jacob got a double dose of an allergy medication.
![Figure][1]
Food allergies are becoming more common, and a handful of foods accounts for the vast majority of allergies. But small doses of the foods can blunt allergic reactions.
PHOTO: SCIENCE PHOTO LIBRARY/SCIENCE SOURCE
The most tragic data point to date is the case in Japan. A child had enrolled in a trial of immunotherapy for milk allergies at the Kanagawa Children's Medical Center in Yokohama. He'd raised what he could ingest from less than 8 milliliters to 135 milliliters—about half a glass of milk. After 3 months on that maintenance dose, he swallowed it and soon complained of pain. Within minutes, he had stopped breathing. His heartbeat was later restored in the emergency room, but he'd gone too long without it and sustained severe brain damage, according to a statement from the hospital's president, Sumimasa Yamashita, in November 2017. Kanagawa Children's Medical Center declined to comment, saying only that the incident remains under investigation.
In its statement, the hospital noted the boy had suffered an asthma attack the day before the catastrophic dose. He also was on a protocol that aimed to rapidly escalate the volume of milk he could drink over less than 3 weeks. But why the child reacted so disastrously to that glass of milk is unknown.
“What people don't understand is this level of protection fluctuates,” says Mimi Tang, a pediatric allergist-immunologist at Murdoch Children's Research Institute in Melbourne, Australia. “It is not guaranteed, nor is it constant.”
One of the few long-term analyses was published in 2013 in The Journal of Allergy and Clinical Immunology . Keet, pediatric allergist-immunologist Robert Wood at Johns Hopkins Medicine, and their colleagues sought out 32 children who'd been in a milk immunotherapy trial. Three to 5 years later, “The results were surprising in a sobering kind of way,” Wood says. Only about a quarter “were doing great … tolerating unlimited quantities of milk without side effects.” Another quarter had abandoned the protocol and returned to strict avoidance. The rest were eating dairy products inconsistently, with intermittent or even frequent allergic reactions. “It's hard to know which comes first, whether they got complacent” about ingesting it “or backed off because [they were] having too many symptoms,” Wood says.
MORE AND MORE families are willing to live with those uncertainties because the alternative is greater anxiety. “We were scared senseless,” says Divya Balachandar, whose daughter Leena Wong, now 7 years old, had her first episode of anaphylaxis at age 4 after being touched by a cashew. Testing revealed Leena also was allergic to sesame, eggs, milk, other tree nuts, and peanuts. Balachandar, a pediatric pulmonologist in New York City, and her husband enrolled Leena in a federally funded oral immunotherapy trial for peanut allergy in 2015. “It made me nervous, really nervous, to put something in my daughter's mouth that she was allergic to,” Balachandar says. She gravitated toward a trial over treatment with a local allergist because, she says, “there were no rules” about how to treat in private practice. By this spring, Leena could eat two spoonfuls of peanut butter—about 25 peanuts—without a problem. She started second grade sitting with her classmates at lunchtime, liberated from a separate nut-free table.
Both companies developing peanut-based treatments say they had more volunteers for their trials than they could accommodate. Private practitioners usually have a waiting list; Greiwe's runs more than 4 months. At Stanford University in Palo Alto, California, which has a large food allergy research program, more than 2000 patients are waitlisted to enroll in the university's clinical trials, says Sharon Chinthrajah, an allergist-immunologist there.
More treatments are on the horizon. In Australia, Tang is working with a company that's testing an approach she pioneered, a combination of a probiotic and oral peanut immunotherapy. The probiotic should tilt the body toward producing the subset of T cells that tolerate the allergen and away from making cells that attack it, she says. Chinthrajah and others are enthusiastic about combining oral immunotherapy with a monoclonal antibody called omalizumab, which is FDA approved to treat allergic asthma. Clinical trials are also gearing up to test other monoclonal antibodies that target molecules involved in allergic inflammation.
Jacob's and Leena's families are eager to see what comes next. Jacob is also allergic to pistachios and cashews, but because he finds those foods easier to avoid than peanuts, the family has rejected immunotherapy that targets them. Leena's family is the opposite. With her older sister and her parents, Leena attends Indian functions regularly, where tree nuts are a common ingredient in sauces. In August, another episode of anaphylaxis landed her in the emergency room: She began to vomit and suffered chest tightness and eye swelling after eating Indian food her parents suspect contained cashews—despite having triple-checked with the restaurant that it did not. “I would love to do tree nuts,” Balachandar says, once immunotherapy “becomes more available and better understood.”
Physicians with deep roots in food allergy immunotherapy hope those new to it tread carefully. Doctors who offer such treatments “have to know the data cold,” including published results and side effects that may crop up, Greenhawt says. Still, he's thrilled that peanut immunotherapy treatments may soon be approved. The other day, talking with a peanut-allergic 4-year-old and his mother, Greenhawt shared what the next year might bring. “I said, ‘I'm going to see you a year from now; hopefully, we will have two products that are approved, and we can talk about which one might be best for you.’” The mother looked startled and delighted, Greenhawt says. “I've never seen somebody smile as brightly as that.”
[1]: pending:yes
Via Krishan Maggon

|
Scooped by
Gilbert C FAURE
October 15, 2018 1:24 PM
|
The alarmin cytokines IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) play a critical role in asthma pathogenesis by inducing mucosal Th2-type cytokine production. Although environmental exposure to aeroallergens has been proposed as an alarmin trigger in asthma, there has been no systematic...

|
Scooped by
Gilbert C FAURE
October 8, 2018 2:33 PM
|
Inhaled environmental allergens elicit type 2 lung inflammation leading to an increase in the risk of developing allergies and asthma. Bankova et al . found that one step along this pathway depends on the lipid mediator leukotriene E4 signaling through a receptor on respiratory epithelial cells to increase the number of brush cells, a rare population of chemosensory cells in the lung epithelium that express receptors shared by taste bud cells. These brush cells were identified as the major pulmonary source for synthesis of interleukin-25 (IL-25), a proinflammatory protein increased in diseases associated with type 2 inflammation. These results highlight the contributions that leukotriene E4 and IL-25 make to the signaling pathways that perpetuate allergic diseases.
Respiratory epithelial cells (EpCs) orchestrate airway mucosal inflammation in response to diverse environmental stimuli, but how distinct EpC programs are regulated remains poorly understood. Here, we report that inhalation of aeroallergens leads to expansion of airway brush cells (BrCs), specialized chemosensory EpCs and the dominant epithelial source of interleukin-25 (IL-25). BrC expansion was attenuated in mice lacking either LTC4 synthase, the biosynthetic enzyme required for cysteinyl leukotriene (CysLT) generation, or the EpC receptor for leukotriene E4 (LTE4), CysLT3R. LTE4 inhalation was sufficient to elicit CysLT3R-dependent BrC expansion in the murine airway through an IL-25–dependent but STAT6-independent signaling pathway. Last, blockade of IL-25 attenuated both aeroallergen and LTE4-elicited CysLT3R-dependent type 2 lung inflammation. These results demonstrate that CysLT3R senses the endogenously generated lipid ligand LTE4 and regulates airway BrC number and function.

|
Scooped by
Gilbert C FAURE
October 4, 2018 8:48 AM
|
We are a European alliance of 41 allergy, asthma and chronic obstructive pulmonary disease (COPD) patients’ associations representing 30% of European citizens currently living with these diseases. Our mission is to convey their voice and to be actively involved in the decisions impacting their...

|
Scooped by
Gilbert C FAURE
September 22, 2018 12:07 PM
|
Preventing Chronic Disease (PCD) is a peer-reviewed electronic journal established by the National Center for Chronic Disease Prevention and Health Promotion. PCD provides an open exchange of information and knowledge among researchers, practitioners, policy makers, and others who strive to...

|
Scooped by
Gilbert C FAURE
September 11, 2018 3:40 AM
|
The alarmin cytokines IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) play a critical role in asthma pathogenesis by inducing mucosal Th2-type cytokine production. Although environmental exposure to aeroallergens has been proposed as an alarmin trigger in asthma, there has been no systematic...

|
Scooped by
Gilbert C FAURE
September 8, 2018 12:43 PM
|
Asthma is a widespread chronic airway disease characterized by airway obstruction, inflammation, and hyperresponsiveness. Symptoms such as bronchoconstriction and cough range from mild intermittent to severe persistent. In eosinophilic asthma, the most common form of asthma, eosinophils in the airway alter nerve function and exacerbate the disease. However, whether eosinophils also affect airway nerve structure is unclear. Now, Drake et al . show that in specimens from patients with severe eosinophilic asthma, airway innervation was increased and positively correlated with symptom severity. In mice, eosinophilia increased airway innervation and triggered bronchoconstriction and airway hyperresponsiveness. The results suggest that structural remodeling of airway innervation contributes to symptom severity in eosinophilic asthma.
In asthma, airway nerve dysfunction leads to excessive bronchoconstriction and cough. It is well established that eosinophils alter nerve function and that airway eosinophilia is present in 50 to 60% of asthmatics. However, the effects of eosinophils on airway nerve structure have not been established. We tested whether eosinophils alter airway nerve structure and measured the physiological consequences of those changes. Our results in humans with and without eosinophilic asthma showed that airway innervation and substance P expression were increased in moderate persistent asthmatics compared to mild intermittent asthmatics and healthy subjects. Increased innervation was associated with a lack of bronchodilator responsiveness and increased irritant sensitivity. In a mouse model of eosinophilic airway inflammation, the increase in nerve density and airway hyperresponsiveness were mediated by eosinophils. Our results implicate airway nerve remodeling as a key mechanism for increased irritant sensitivity and exaggerated airway responsiveness in eosinophilic asthma.

|
Scooped by
Gilbert C FAURE
August 20, 2018 5:00 AM
|
Our on-demand webinar discusses the top allergy and asthma codes billed by providers and how to effectively data mine for patterns and schemes....
The latest rage in treatment for allergic disease is a class of biologic drugs called monoclonal antibodies. They are miracle drugs for those who have trouble controlling asthma and eczema. They are also very expensive.
Via Krishan Maggon

|
Scooped by
Gilbert C FAURE
August 3, 2018 2:35 PM
|
The importance of developing new animal models to assess the pathogenesis of glucocorticoid (GC)-insensitive asthma has been stressed. Because of the asthma-prone background of A/J mice, we hypothesized that asthma changes in these animals would be or become resistant to GCs under repeated exposures to an allergen. A/J mice were challenged with OVA for 2 or 4 consecutive d, starting on day 19 postsensitization. Oral dexamethasone or inhaled budesonide were given 1 h before challenge, and analyses were done 24 h after the last challenge. Airway hyperreactivity, leukocyte infiltration, tissue remodeling, and cytokine levels as well as phosphorylated GC receptor (p-GCR), p-GATA-3, p-p38, MAPK phosphatase-1 (MKP-1), and GC-induced leucine zipper (GILZ) levels were assessed. A/J mice subjected to two daily consecutive challenges reacted with airway hyperreactivity, subepithelial fibrosis, and marked accumulation of eosinophils in both bronchoalveolar lavage fluid and peribronchial space, all of which were clearly sensitive to dexamethasone and budesonide. Conversely, under four provocations, most of these changes were steroid resistant. A significant reduction in p-GCR/GCR ratio following 4- but not 2-d treatment was observed, as compared with untreated positive control. Accordingly, steroid efficacy to transactivate MKP-1 and GILZ and to downregulate p-p38, p-GATA-3 as well as proinflammatory cytokine levels was also seen after two but not four provocations. In conclusion, we report that repeated allergen exposure causes GC-insensitive asthma in A/J mice in a mechanism associated with decrease in GCR availability and subsequent loss of steroid capacity to modulate pivotal regulatory proteins, such as GATA-3, p-p38, MKP-1, and GILZ.

|
Scooped by
Gilbert C FAURE
July 29, 2018 2:44 AM
|
IL-1 family regulatory cytokine IL-37b can suppress innate immunity and inflammatory activity in inflammatory diseases. In the present study, IL-37b showed remarkable in vitro suppression of inflammatory TNF-α, IL-1β, IL-6, CCL2 and CXCL8 production in the co-culture of human primary eosinophils and human bronchial epithelial BEAS-2B cells with the stimulation of bacterial toll-like receptor-2 (TLR2) ligand peptidoglycan (PGN), while antagonizing the activation of intracellular nuclear factor-κB, PI3K-Akt, extracellular signal–regulated kinase (ERK)1/2 and suppressing the gene transcription of allergic inflammation-related PYCARD, S100A9 and CAMP as demonstrated by flow cytometry, RNA-sequencing and bioinformatics. Results therefore elucidated the novel anti-inflammation-related molecular mechanisms mediated by IL-37b. Using the house dust mite-induced humanized asthmatic NOD/SCID mice for pre-clinical study, intravenous administration of IL-37b restored the normal plasma levels of eosinophil activators CCL11 and IL-5, suppressed the elevated concentrations of Th2 and asthma-related cytokines IL-4, IL-6 and IL-13 and inflammatory IL-17, CCL5 and CCL11 in lung homogenate of asthmatic mice. Histopathological results of lung tissue illustrated that IL-37b could mitigate the enhanced mucus, eosinophil infiltration, thickened airway wall and goblet cells. Together with similar findings using the ovalbumin- and house dust mite-induced allergic asthmatic mice further validate
|




 Your new post is loading...
Your new post is loading...













![[Policy meeting of the European Parliament Interest Group on Allergy and Asthma] How to overcome the barriers to research in chronic NCDs in Europe? The case of allergy and asthma | Allergy (and clinical immunology) | Scoop.it](https://img.scoop.it/RPVVRgJg0-yfmQpuhljmczl72eJkfbmt4t8yenImKBVvK0kTmF0xjctABnaLJIm9)










