Researchershave prevented the binding of peanut allergens with IgE to suppress the allergic reaction to peanuts using novel allergen-specific inhibitors.
Get Started for FREE
Sign up with Facebook Sign up with X
I don't have a Facebook or a X account


 Your new post is loading... Your new post is loading...
 Your new post is loading... Your new post is loading...
IgE‐mediated food allergy (FA) is a potentially life‐threatening condition with a negative impact on quality of life and an increasing prevalence in westernized countries in the recent two decades. A strict avoidance of the triggering food(s) represents the current standard approach. However, an elimination diet may be difficult and frustrating, in particular for common foods, (e.g. milk, egg, and peanut). Food allergy immunotherapy (FA‐AIT) may provide an active treatment that enables to increase the amount of food that the patient can intake without reaction during treatment (i.e. desensitization), and reduces the risk of potential life‐threatening allergic reaction in the event of accidental ingestion. However, several gaps need still to be filled. A memorable Latin orator stated: “Est modus in rebus” (Horace, Sermones I, 1, 106‐07). This sentence remembers that there is a measure in everything to a proper proportion of therapy. The common sense of measure should find application in each stage of treatment. A personalized approaching should consider the specific willing and features of each patient. Efforts are devoted to improve the efficacy, the safety but also the quality of life of patients suffering from FA. In the near future it will be important to clarify immunological pathways of FA‐AIT, and to identify reliable biomarkers in order to recognize the most suitable candidates to FA‐AIT and algorithms for treatments tailored on well‐characterized subpopulations of patients. This article is protected by copyright. All rights reserved. Via Krishan Maggon
Clin Exp Allergy. 2019 Jan 28. doi: 10.1111/cea.13348.[Epub ahead of print]...
<b><i>Background:</i></b> Peanut storage proteins (Ara h 1, Ara h 2, and Ara h 3) have been described as the major peanut allergens in children, although not all peanut-sensitized individuals have cli...
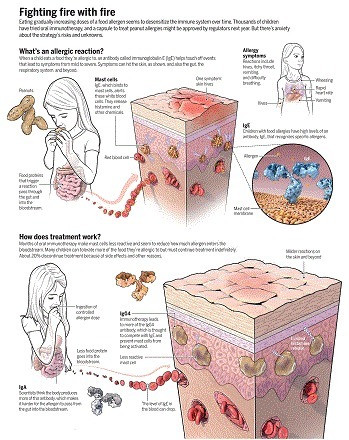
Changes in the human environment and activities over the past few decades have caused an epidemic of food allergies ([ 1 ][1]). People suffering from allergies often feel that they live on a cliff edge, as the allergens to which they react are potentially fatal ([ 2 ][2]). For example, tiny amounts of peanut picked up on skin or contaminating other foods can be dangerous to peanut-sensitized individuals ([ 2 ][2]–[ 4 ][3]). Immunoglobulin E (IgE) antibodies mediate the allergic response. They bind to specific receptors on inflammatory immune cells: mast cells in mucosal tissues lining body surfaces and cavities, and basophils in the circulation. These cells mediate allergic responses triggered by specific antigens (allergens) that are recognized by IgE. B cells expressing IgG antibodies have long served as the paradigm for the development of B cells into antibody-secreting plasma cells in the immune response. Until recently, the far less abundant IgE-expressing B cells have proved to be elusive. On page 1306 of this issue, Croote et al. ([ 5 ][4]) have analyzed single B cells from six individuals with peanut allergy, which enabled the identification of the natural Ig heavy- and light-chain pairs from IgE-expressing B cells that are responsible for peanut allergy. With this information they produced recombinant antibodies, identified the peanut allergen–specific antibodies, and used site-directed mutagenesis to suppress their activity. The mutated antibodies could be used to treat peanut allergy.
Original Article from The New England Journal of Medicine — AR101 Oral Immunotherapy for Peanut Allergy...
In this phase 3 trial of oral immunotherapy in children and adolescents who were highly allergic to peanut, treatment with AR101 resulted in higher doses of peanut protein that could be ingested without dose-limiting symptoms and in lower symptom severity during peanut exposure at the exit food challenge than placebo. (Funded by Aimmune Therapeutics; PALISADE ClinicalTrials.gov number, NCT02635776.) Via Krishan Maggon
Ingesting small doses of peanut products guards against allergic reactions—but an undercurrent of anxiety persists. Via Krishan Maggon
Back to listingNews Scientists develop a new test to safely and accurately diagnose peanut allergies 3 May 2018 MRC scientists have developed a new laboratory test to diagnose peanut allergy. The test has 98% specificity and, unlike current options, it doesn’t run the risk of false-positives or causing allergic reactions such as anaphylactic shock. The simple blood test is five times more cost-efficient compared to the oral food challenge (OFC) – the standard food allergy test – and could be adapted to test for other food allergies. Peanut allergies are among the most common food allergies in children*. Currently, doctors diagnose peanut allergy using a skin-prick test or specific IgE test but this may result in over-diagnosis or false-positives and it cannot differentiate between sensitivity and true food allergy. When skin-prick and IgE test results are unclear, allergists rely on an OFC, which consists of feeding peanut in incrementally larger doses to a patient in a highly-controlled setting in hospital to confirm allergy to the food. While the test is the gold-standard for diagnosing food allergies, there is risk of causing severe allergic reactions. Now, the researchers have developed a safer, accurate blood test in the lab. The new test, called the mast activation test (MAT), could act as a second line tool when skin-prick test results are inconclusive and before referring children and their families to specialists for an OFC, according to researchers from the MRC & Asthma UK Centre in Allergic Mechanisms of Asthma. Their new study was published in the Journal of Allergy and Clinical Immunology. Dr Alexandra Santos, an MRC Clinician Scientist at King’s College London, paediatric allergist and study lead author, said: “The current tests are not ideal. If we relied on them alone, we’d be over diagnosing food allergies – only 22% of school-aged children in the UK with a positive test to peanuts are actually allergic when they’re fed the food in a monitored setting.” Dr Santos continued: “The new test is specific in confirming the diagnosis so when it’s positive, we can be very sure it means allergy. We would reduce by two-thirds the number of expensive, stressful oral food challenges conducted, as well as saving children from experiencing allergic reactions.” Food allergy symptoms are triggered when allergens interact with an antibody called immunoglobulin E (or IgE). The food allergens activate IgE antibodies, triggering symptoms such as skin reactions, itching or constricting of the mouth, throat and airways, and digestive problems (such as stomach cramps, nausea or vomiting). The current skin-prick test and IgE test, which have been in use for decades, measure the presence of IgE antibodies. The new test focuses on mast cells, which play a crucial role in triggering allergic reactions. Mast cells activate by recognising the IgE in plasma and, in allergic patients, produce biomarkers associated with allergic reactions, which can be detected in the lab. Using blood samples from 174 children participating in allergy testing – 73 peanut allergic and 101 peanut-tolerant – the scientists added peanut protein to mast cells to screen for IgE-mediated activation. The MAT accurately identified peanut allergy with 98 specificity. (Specificity is a statistical measure in determining efficacy for diagnosis. The MAT test rarely gives positive results in non-allergic patients.) The researchers also found the test reflected the severity of peanut allergy – patients with more severe reactions have a higher number of activated mast cells. The MAT test is five times cheaper to conduct than the OFC, which requires an allergist and specialist nurses on hand to monitor for adverse reactions and provide medical support if symptoms arise. Dr Santos said: “We are adapting this test to other foods, such as milk, eggs, sesame and tree nuts. This test will be useful as we are seeing more and more children who have never been exposed to these foods because they have severe eczema or have siblings with allergies. Parents are often afraid to feed them a food that is known to cause allergic reactions.” The researchers believe the MAT test may have other uses, for example, in the food industry to detect the presence of allergens in products. Pharmaceutical companies could use it to monitor patients’ allergic response to drugs being evaluated during clinical trials. The scientists plan to transition the biomarker test out of the laboratory and into a clinical setting. They will be testing blood samples from patients with suspected allergies to further validate its utility. 5 to 8% of UK children have a food allergy with up to one in 55 children having a peanut allergy, according to Food Standards Agency estimates. Current UK guidelines recommend avoiding giving your child peanuts and foods containing peanuts before the age of six months. Other countries, such as Canada and the United States, have updated their recommendations – a move that is in the works in the UK. The researchers say updated guidelines may result in a rise in requests for peanut allergy diagnosing. This paper is available on Europe PMC. Categories Categories: Research Health categories: Blood, Inflammatory, Respiratory, Generic Strategic objectives: Lifestyles affecting health, Environment and health, Securing impact from medical research, Aim: Picking research that delivers, Aim: Research to people, Aim: Supporting scientists Locations: London Type: News article
Food allergy is increasingly common in children, affecting about 4%‐8%. The mainstays of management remain allergen avoidance and emergency preparedness to treat allergic reactions with emergency medications. Unfortunately, these approaches are unsatisfactory for many patients and their families as the restrictions, constant vigilance, and unpredictable severity of allergic reactions negatively impact quality of life. In recent decades, there has been significant interest in developing treatments for food allergy that lead to desensitization to increase thresholds for triggering allergic reactions and decrease the risk of reacting to allergen‐contaminated food products. Epicutaneous immunotherapy (EPIT) is a novel therapy that is currently under investigation, delivering allergen via repeated applications to the skin and targeting antigen‐presenting cells in the superficial skin layers. Murine models have demonstrated that allergen uptake is an active process by skin dendritic cells with subsequent migration to draining lymph nodes. Allergen exposure to the non‐vascularized epidermis limits systemic absorption, contributing to the high‐safety profile. Results from murine experiments showed that EPIT has comparable efficacy as subcutaneous immunotherapy in terms of challenge outcomes, airway hyper‐responsiveness, and immunologic parameters. Several clinical trials of EPIT have recently been completed or are ongoing. Results support the high safety and tolerability of this approach. Efficacy data suggest that the change in threshold eliciting dose following 1 year of therapy is less than that seen compared to high‐dose (2‐4 g peanut protein) oral immunotherapy, but more prolonged treatment with EPIT appears to lead to increasing desensitization. Additional data from larger‐scale studies should provide a more robust assessment of safety and efficacy of EPIT. Via Krishan Maggon
Peanuts are the most common food to provoke fatal or near-fatal anaphylactic reactions.
BRISBANE, Calif.--(BUSINESS WIRE)--Feb. 20, 2018-- Aimmune Therapeutics, Inc. (Nasdaq:AIMT), a biopharmaceutical company developing treatments for potentially life-threatening food allergies, today announced that its pivotal Phase 3 PALISADE efficacy trial of AR101 met the primary endpoint. In the United States, AR101 has U.S. Food and Drug Administration (FDA) Breakthrough Therapy Designation for peanut-allergic patients ages 4–17.AR101 has U.S. Food and Drug Administration (FDA) Breakthrough Therapy Designation for peanut-allergic patients ages 4–17.
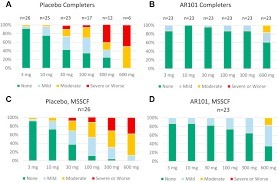
PALISADE (Peanut ALlergy Oral Immunotherapy Study of AR101 for DEsensitization in Children and Adults) is our core, pivotal Phase 3 clinical trial for AR101. PALISADE was the largest randomized clinical trial for peanut allergy to date, enrolling more than 550 participants ages 4-55 in the U.S., Canada, and Europe. Landmark 554-patient Phase 3 study met the primary efficacy endpoint, as 67.2% of AR101 patients ages 4–17 tolerated at least a 600-mg dose of peanut protein in the exit food challenge, compared to 4.0% of placebo patients (p<0.00001) The lower-bound of the 95% confidence interval (CI) of the difference between treatment arms at the primary endpoint was 53.0%, greatly exceeding the pre-specified threshold of 15% (p<0.00001) 50.3% of AR101 patients ages 4–17 tolerated a 1000-mg dose of peanut protein in the exit food challenge, compared to 2.4% of placebo patients (p<0.00001) Among patients ages 4–17 who completed treatment with AR101, 96.3% tolerated a 300-mg dose of peanut protein in the exit food challenge, 84.5% tolerated a 600-mg dose, and 63.2% tolerated a 1000-mg dose 79.6% of AR101 patients ages 4–17 completed the trial; of the 20.4% who discontinued treatment, 12.4% withdrew due to treatment-related adverse events 2.4% of AR101 patients ages 4–17 and 0.8% of placebo patients experienced serious adverse events Via Krishan Maggon 
Krishan Maggon 's curator insight,
February 21, 2018 3:40 AM
AR101 has U.S. Food and Drug Administration (FDA) Breakthrough Therapy Designation for peanut-allergic patients ages 4–17.
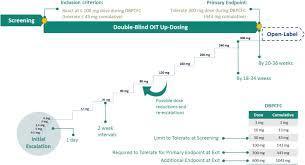
Gradually increasing doses of AR101 would desensitize patients to peanut over a period of about six months. Afterward, patients would continue to take maintenance doses of AR101 in order to maintain desensitization.
<b><i>Background:</i></b> Little is known about breast milk as a vehicle for tolerance development or sensitization to peanuts very early in life. Thus, well-characterized and
The vast majority of children in a large clinical trial continued to tolerate peanuts for 4 years after it had ended, new research shows. |
This randomized clinical trial compares the efficacy and safety of epicutaneous immunotherapy with a peanut patch vs placebo among peanut-allergic children.
DBV Technologies is jumping back into the race to bring an FDA-approved peanut allergy treatment to the market, but it might not be enough to beat its...
Original Article from The New England Journal of Medicine — Effect of Avoidance on Peanut Allergy after Early Peanut Consumption...
CME / ABIM MOCPeanut Allergy: Pathophysiologic Mechanisms and Therapeutic Options
Abstract All body surfaces are exposed to a wide variety of microbes, which significantly influence immune reactivity within the host. This review provides an update on some of the critical novel findings that have been published on the influence of the microbiome on atopic dermatitis, food allergy and asthma. Microbial dysbiosis has consistently been observed in the skin, gut and lungs of patients with atopic dermatitis, food allergy and asthma, respectively, and the role of specific microbes in allergic disorders is being intensively investigated. However, many of these discoveries have yet to be translated into routine clinical practice. Abbreviations AAI allergic airway inflammation AD atopic dermatitis AHR airway hyper‐responsiveness AMP antimicrobial peptides COPD chronic obstructive pulmonary disease CRS chronic rhinosinusitis GCS glucocorticoids HDM house dust mite HMOs human milk oligosaccharides ICSs inhaled corticosteroids LABAs long‐acting β2 adrenergic receptor agonists OIT oral immunotherapy PARs protease‐activated receptors PSMs phenol‐soluble modulins RSV respiratory syncytial virus SCFAs short‐chain fatty acids TLR Toll‐like receptor 1 INTRODUCTION An enormous variety of microbes colonize the skin and mucosal body surfaces. These microbes are organized within complex community structures, utilizing nutrients from other microbes, host secretions and the diet. The microbiome is defined as the sum of these microbes, their genomic elements and interactions in a given ecological niche. In addition to bacteria, viruses are also considered to be an important component of the microbiome (virome). The composition of the microbiome is dependent on the specific body site examined, resulting in a series of unique habitats within and between individuals that can change substantially over time.1 This presents significant challenges to the local immune system, which should tolerate the presence of these microbes to avoid damaging host tissue while retaining the ability to respond appropriately to pathogens. The mechanisms that mediate host‐microbe communication are highly sophisticated and need to be constantly coordinated.2 Indeed, disrupted communication between the microbiome and the host due to altered microbiome composition and/or metabolism is thought to negatively influence immune homeostatic networks and may play a role in immune hypersensitivity to environmental exposures, such as allergens.3-5 For several years, epidemiological studies have suggested associations between the migration from traditional farming to urban environments, increase in processed food intake, lack of contact with animals and excessive hygiene practices with the increased incidence of asthma, atopic dermatitis and food allergy. However, it is only relatively recently that the importance of the gut, lung and skin microbiomes in regulation of immune tolerance and its aberrations in a variety of human diseases including allergy and asthma has been recognized.6, 7 In particular, early‐life events such as mode of delivery, breastfeeding, mother's diet and health status, antibiotics and other drug usage in pregnancy and early childhood, early‐life environment (ie, siblings, pets at home, proximity to farm animals and green areas) significantly influence the timing of bacterial colonization and establishment, which modify the risk of developing allergies and asthma, as summarized in Figure 1.8-17 In this review, we will highlight some of the recent advances in our knowledge regarding the influence of the microbiome on immune reactivity in the skin, gut and lungs of patients with atopic dermatitis, food allergy and asthma. In addition, we will discuss the potential translation and challenges associated with microbial‐based therapies in patients with these allergic disorders. 2 MICROBIOME IN ATOPIC DERMATITIS The skin microbiome is comprised of bacteria, fungi, viruses and archaeal communities, with bacteria being the most widely studied.18 The skin microbiome is influenced by age, gender, ethnicity, climate, UV exposure and lifestyle factors.19 16S ribosomal RNA (rRNA) sequencing has demonstrated that significantly diverse bacterial phyla exist on healthy skin with site‐specific differences in composition. This is primarily driven by the physiology of a skin niche. Propionibacterium species are predominantly found in sebaceous sites, with Corynebacterium and Staphylococcus species occurring in moist microenvironments. Malassezia represents the predominant fungal flora on human skin.20 Figure 2 illustrates the interactions between the skin microbiome and host cells. Atopic dermatitis (AD) is characterized by epidermal barrier dysfunction resulting from a synergistic decrease in epidermal barrier structural proteins, alteration in lipid composition and skin pH, activation of local and systemic inflammatory responses and decrease in skin microbiome diversity.19 Staphylococcus aureus overgrowth is consistently linked with AD pathogenesis and correlates with disease severity and eczematous flares.1, 21 High IL‐4 and IL‐13 levels within AD skin can deplete keratinocyte‐produced antimicrobial peptides (AMPs), cathelicidin LL‐37, human beta defensin hBD‐2 and hBD‐3, necessary for controlling pathogenic organisms.22 Defective TLR‐2 expression in Langerhans cells of AD skin has also been observed, which may contribute to the impairment in effective immune recognition and clearance of pathogenic bacteria such as S. aureus.23 Epidermal lipid composition strongly correlates with bacterial diversity and composition at typical sites for AD lesions. For example, S. aureus dominance was associated with elevated levels of ceramide AS.21 Staphylococcus aureus overgrowth with concomitant decline in Staphylococcus epidermidis is a general feature of AD and is not restricted to eczematous lesions.19, 21 Staphylococcus aureus colonization is evident in 90% of AD cases,24 associates with AD severity and increased allergen sensitization.25 Intervention studies with antimicrobials targeting S. aureus can reduce AD severity. Restoration of the epithelial barrier with anti‐inflammatory and emollient use is able to increase microbial diversity of lesional skin.1, 24 Patients with severe AD can be colonized with a single S. aureus strain, which persists even post‐eczematous flare albeit at a lower relative abundance. In contrast, S. epidermidis strains were more heterogeneous. Interestingly, patients with more severe AD were colonized with methicillin‐sensitive staphylococci, whereas less severe AD was more frequently associated with methicillin‐resistant strains. This observation may have significant treatment implications, particularly when methicillin‐sensitive S. aureus and methicillin‐resistant S. epidermidis strains are present.26 In a recent study, the skin microbiome of infants with AD showed a consistent absence of S. aureus sequences at multiple time points on lesional skin contrary to reported finding in patients with established AD. The most prevalent species were S. epidermidis and S. cohnii. However, those who developed AD at 12 months had significantly lower levels of these commensal staphylococci detectable at 2 months of age.27 This study suggests that S. aureus colonization may not always predate clinical AD and highlights the need for longitudinal studies to investigate the transition to microbial dysbiosis in AD. Commensal S. epidermidis strains can also increase during disease flares.24 Coagulase‐negative staphylococci (CoNS), which include S. epidermidis, S. hominis and S. lugdunensis, can secrete antimicrobials that limit S. aureus overgrowth and biofilm formation.1, 28 In addition, S. epidermidis activates TLR2, thereby promoting tight junction protein expression and inducing keratinocyte‐derived antimicrobial peptide secretion. Early occupation of the neonatal human skin by S. epidermidis is associated with induction of S. epidermidis‐specific FOXP3+ Treg cells that regulate local activation of host immune responses.29 Other members of the healthy skin microbiota, such as Propionibacterium, Streptococcus, Acinetobacter, Corynebacterium, Prevotella and Proteobacteria, are frequently reduced in AD patients.28, 30 Staphylococcus aureus can contribute to epidermal barrier disruption in a number of ways. Staphylococcus aureus downregulates terminal differentiation proteins such as filaggrin and loricrin, while secretion of proteases contributes to the disruption of the epidermal integrity via direct proteolytic activity or activation of protease‐activated receptors (PARs). Superantigens such as staphylococcal enterotoxins A and B or toxic shock syndrome toxin‐1 trigger a cytokine response that further disrupts the epidermal barrier. These enterotoxins also act as allergens, and toxin‐specific IgE contributes to cutaneous inflammation.28, 31 Staphylococcus aureus expresses exotoxins such as cytolytic α‐toxin, which damage keratinocytes, while β‐, γ‐ and δ‐toxins stimulate mast cell degranulation.28, 32 Phenol‐soluble modulins (PSMs) induce keratinocyte damage and secretion of the alarmins IL‐1α and IL‐36α, which further exaggerate skin inflammation.33 An impaired skin barrier results in increased exposure of the immune system to microbial components, resulting in a progressive cycle of inflammatory responses and tissue damage. It was recently suggested that reactivity to S. aureus can be facilitated via allergen co‐exposure and vice versa since patients with sensitization to house dust mite also show significantly more IgE reactivity to S. aureus and Escherichia coli, two abundant species in the house dust mite microbiome.34 A subset of AD patients is susceptible to eczema herpeticum (EH), and S. aureus may contribute to EH susceptibility as it has been shown to secrete products that enhance viral replication.1 Despite Malassezia species having a commensal role in healthy skin, in AD Malassezia may contribute to disease pathogenesis. Malassezia DNA has been detected in 90% of AD skin lesions, and colonization increases with disease severity.35 In addition, different Malassezia strains were found in AD and healthy individuals suggesting the existence of key pathogenic strains in AD.36 Higher levels of IgE sensitization to Malassezia have been detected in adult AD compared to healthy individuals and childhood AD.22, 36 Malassezia could contribute to AD pathogenesis by secreting immunogenic proteins that induce proinflammatory cytokines, expression of TLR2 and TLR4 on keratinocytes and induction of auto‐reactive T cells.22 Atopic dermatitis is considered a first step in the atopic diathesis, facilitated in part by the defective epidermal barrier of AD. The IL‐4/IL‐13 axis in AD is also thought to upregulate the pore‐forming claudin‐2 expression in the gut leading to barrier defects.19 In addition to the skin microbiota, AD has been associated with changes in the gut microbiota. Patients with AD have lower levels of Bifidobacterium in the gut compared to healthy controls, and Bifidobacterium levels were inversely correlated with AD disease severity.37 Several studies have shown that alterations in gut microbiota composition can precede the development of AD. Early gut colonization with C. difficile was associated with AD development,38 and low gut microbiota diversity and specifically low Bacteroidetes diversity at 1 month were associated with AD development at 2 years of age.35, 39 A recent whole‐metagenome analysis demonstrated a lower abundance of key metabolic pathways in AD children associated with depletion of mucin‐degrading bacteria such as Akkermansia muciniphila, Ruminococcus gnavus and Lachnospiraceae.40 These bacteria not only are able to influence immune development through directly influencing signalling pathways and antigen processing but also can lead to a reduced microbial diversity as these bacteria are able to degrade complex polysaccharides into short‐chain fatty acids (SCFAs)—nutrient sources that allow for gut colonization by other microbes.40 Dog exposure at birth was associated with a dose‐related reduced risk of AD in early life, suggesting that exposure to an environment rich in microbial components may be protective.41 In contrast, antibiotic exposure during the first 2 years of life is associated with an increased risk of AD.42 Infants with high faecal calprotectin levels (an antimicrobial protein used as a biomarker of intestinal inflammation) measured at 2 months of age had an increased risk of AD and asthma by 6 years of age. High faecal calprotectin was also shown to be inversely correlated with levels of E. coli. Reduced early colonization with E. coli was shown to impair IL‐10 regulation.43 3 MICROBIOME IN FOOD ALLERGY The human gut microbiome is increasingly being considered as a crucial factor in the development of food allergy, with a strong interrelation between the human gut microbiota, environmental factors, human genetics and gastrointestinal atopy.4, 44 In particular, the composition and metabolic activity of the gut microbiota are intimately linked with the development of oral tolerance.45, 46 Therefore, disturbed microbial homeostasis, especially early in life, appears to significantly influence allergic disease susceptibility. Figure 3 illustrates some of the known interactions between the gut microbiome and host mucosal cells. Recently, the oral bacterial composition in saliva samples from healthy and allergic children up to 7 years of age was described. The result confirmed that early changes in oral microbial composition seem to associate with immune maturation and allergy development.47 Milk‐allergic infants have higher total bacteria and anaerobic bacterial counts compared with healthy control children after 6 months of differential formula intake. In addition, higher proportions of Lactobacilli and lower proportions of Enterobacteria and Bifidobacteria were observed in 46 milk‐allergic infants.48 The spontaneous resolution of milk allergy in infants was associated with a specific gut microbiota composition.49 Bunyavanich et al showed that Clostridia and Firmicutes were enriched in the infant gut microbiome of subjects whose milk allergy spontaneously resolved. This result suggested that early infant gut microbiota may shape food allergy outcomes in childhood and bacterial taxa within Clostridia and Firmicutes species could be further investigated as probiotic candidates for milk allergy therapy.49 An additional study examining the gut microbiome of 141 children with egg allergy and healthy controls found that genera from Lachnospiraceae and Ruminococcaceae were associated with egg sensitization; however, there was no association between early‐life gut microbiota and egg allergy resolution by age 8 years.50 A prospective microbiome association study in 14 children with food allergy and 87 children with food sensitization showed that the genera Haemophilus, Dialister, Dorea and Clostridium were underrepresented among subjects with food sensitization, whereas the genera Citrobacter, Oscillospira, Lactococcus and Dorea were underrepresented among subjects with food allergy.51 An additional prospective study identified both temporal variation and long‐term variation in the differential abundance of specific bacterial genera in children developing IgE‐associated allergic disease, with Faecalibacterium correlating with IL‐10 and Foxp3 mRNA levels.52 Human milk oligosaccharides (HMOs) have been shown to be important in supporting the establishment of the infant gut microbiome as they are selective substrates for protective microbes such as Bifidobacteria.53 Two recent studies have described differences in HMO composition that are associated with cow's milk allergy or food sensitization.54, 55 One potential mechanism for this association is that different HMO profiles may support the establishment of different microbes early in life, thereby indirectly influencing immune maturation and education. In conclusion, a number of human studies now suggest that food allergy could be associated with changes in microbial exposures in early life, which modifies the development of host immunity and results in pathologic immune responses to food allergens. 4 MICROBIOME IN ASTHMA Composition of the microbiome at all mucosal sites changes dynamically in the first days, months and years of life. If the process of “healthy” and timely colonization is disrupted, the early‐life dysbiosis of the gut and lung becomes an important risk factor for atopy, allergy and asthma. In the Canadian Healthy Infant Longitudinal Development (CHILD) study, the lower relative abundance of the bacterial genera Lachnospira, Veillonella, Faecalibacterium and Rothia in the gut was associated with the development of asthma later in life and mechanistically linked with the reduced levels of faecal SCFAs.56 Another recent study also showed that high levels of SCFAs early in life were protective against later life sensitization and asthma.57 In a US birth cohort, lower relative abundance of Bifidobacterium, Akkermansia and Faecalibacterium, with higher relative abundance of Candida and Rhodotorula, in the gut of neonates significantly increased the risk of developing multisensitized atopy and asthma later in life.58 Interestingly, the faecal metabolome of those children at increased risk contained increased levels of pro‐inflammatory metabolites, among which 12, 13‐DiHOME was able to induce IL‐4 production in CD4+ T cells and decreased the abundance of Tregs.58 Increased abundance of nasopharyngeal Lactobacillus species during acute respiratory infection with respiratory syncytial virus (RSV) in infancy was associated with reduced risk of wheezing at 2 years of age.59 Colonization of the airways with Streptococcus, Moraxella or Haemophilus within the first 2 months of life was associated with virus‐induced acute respiratory infections in the first 60 weeks of life as well as increased risk of asthma later in life.60 Colonization of the hypopharynx within the first month of life with Moraxella catarrhalis, Haemophilus influenzae or Streptococcus pneumoniae was associated with low‐grade systemic inflammation as assessed by serum CRP, TNF‐alpha and IL‐6 levels.61 In addition, a positive association was observed between RSV infection and hospitalization in children with nasopharyngeal colonization with H. influenzae and Streptococcus.62, 63 Importantly, the relative nasopharyngeal abundance of Streptococcus and Staphylococcus negatively correlated with FEV1 and PC20 in children.64 Children who were breastfed and those who had low rates of respiratory infections in the first 2 years of life were colonized early within the upper respiratory tract with Staphylococcus species, followed by Corynebacterium, Dolosigranulum and Moraxella.65-67 However, the most impressive data regarding asthma protection have been observed in relation to traditional farming environments, associated with a high endotoxin and bacterial‐containing dust within the home.5, 15, 17, 68-71 Adult asthma patients treated with inhaled corticosteroids (ICSs) have greater upper and lower airway microbiota diversity compared to control subjects, especially enriched in the phylum Proteobacteria, which include Haemophilus, Comamonadaceae, Sphingomonadaceae, Nitrosomonadaceae, Oxalobacteraceae and Pseudomonadaceae families.72-76 The phylum Proteobacteria is also associated with worse asthma control, whereas Actinobacteria correlates with improvement or no change in asthma control.77 Interestingly, neutrophilic exacerbations of asthma and chronic obstructive pulmonary disease (COPD) correlated with the presence of Proteobacteria in the sputum, whereas eosinophilic exacerbations correlated with the presence of Bacteroidetes.78 Mycoplasma pneumoniae and Chlamydophila pneumoniae are also often found in the airways of the severe asthmatic.79 Macrolide antibiotic treatment may be useful in this subgroup of patients, but patients should be carefully selected.80 Both clarithromycin and azithromycin have been shown to reduce airway hyper‐responsiveness and decrease the abundance of Pseudomonas, Haemophilus and Staphylococcus,73, 81 while increasing the relative abundance of Streptococci.82 However, it is currently not clear how significant a role asthma medications play in directly influencing the composition of the airway microbiota. It has been reported that combination of ICS and oral glucocorticoids (GCS) correlates positively with the increased abundance of Proteobacteria, specifically Pseudomonas, and with a decreased abundance of Bacteroidetes, Fusobacteria and Prevotella.83 In corticosteroid‐resistant patients, Neisseria‐Haemophilus, Campylobacter and Leptotrichia species are present in the lower airways.75 Interestingly, treatment of COPD patients with ICS and long‐acting β2 adrenergic receptor agonists (LABAs), compared to LABA alone, significantly increased the bacterial load, increased bacterial diversity and changed composition of the microbiome in the airways.84 However, prospective longitudinal studies involving corticosteroid‐naïve asthma patients are still needed to address the issue of medication effects on the airway microbiome. The mechanisms responsible for changes in the airway microbiome are also not well understood, and in addition to medications, it is possible that the type of inflammatory response (ie, eosinophil vs neutrophil), changes in host secretions (eg, lipids85, 86) and cellular metabolism might influence microbial colonization and growth within the airways. Figure 4 illustrates the immune responses in the airways that can be influenced by the respiratory microbiome. In addition to asthma, the potential for microbes to play a role in the initial aetiology of rhinitis, or in exacerbations and progression to more severe inflammatory sequelae (such as asthma) is currently being examined. The phylum Proteobacteria is enriched in children with rhinitis, which may be clinically important given the Proteobacteria‐related asthma associations described above.69 Dysbiosis of the inferior turbinate mucosa microbiota, particularly an increase in S. aureus and a decrease in P. acnes, was associated with high total IgE levels in adults with allergic rhinitis.87 In adults with chronic rhinosinusitis (CRS), the genus Corynebacterium was depleted, accompanied by increased relative abundance of genera from the phyla Firmicutes (including Staphylococcus and Streptococcus), Proteobacteria (including Haemophilus, Pseudomonas and Moraxella) or Fusobacteria. This trend was particularly evident in subjects with comorbidities such as asthma and cystic fibrosis.88 Similarly, another study reported that middle meatus samples from CRS patients without nasal polyps were enriched in Streptococcus, Haemophilus and Fusobacterium but exhibited loss of diversity compared to healthy, CRS with nasal polyps and allergic rhinitis subject samples.89 5 LEARNING FROM ANIMAL MODELS Despite the compelling observations and associations in humans that link changes in the microbiota with allergic diseases, very often the causal relationship is not clear. Microbial dysbiosis can be the reason for the disease but can also be the consequence of inappropriate immune reactivity. Animal models have been used to better understand the role of microbes in directly influencing allergic diseases and to elucidate the molecular mechanisms underpinning host‐microbe crosstalk. 5.1 Atopic dermatitis Similar to humans, dogs naturally develop AD and associated allergen sensitization. Canine AD is associated with reduced bacterial diversity, with increased abundance of Staphylococcus pseudintermedius and Corynebacterium species.90 Canine AD lesions improve with antimicrobial treatment and a reduction in Staphylococcus species coincided with restoration of bacterial diversity.30 Filaggrin‐deficient flaky tail mice carry a loss‐of‐function filaggrin mutation, which is associated with a defective epidermal barrier, epidermal hydration and flexibility. Staphylococcus aureus abundance on the skin of these mice correlates with Th2 cytokine levels.91 Inbred DS‐Ng mice develop spontaneous dermatitis, and the skin lesions have been shown to be heavily colonized by S. aureus.29 Staphylococcus aureus triggered cutaneous inflammation involve the accessory gene regulatory (Agr) virulence systems of S. aureus and induced δ‐toxin molecules, which initiate Th2 type skin inflammation. Targeted S. aureus and Corynebacterium bovis antimicrobial therapy improved eczematous lesions and increased bacterial diversity in Adam 17 (a transmembrane metalloproteinase)‐deficient mice. Withdrawal of targeted antimicrobials resulted in a recurrence of eczema and microbial dysbiosis.30 In a mouse itch model, IL‐17A and IL‐22 drive neutrophils to limit the overgrowth of S. aureus on injured skin.25 C5aR‐deficient mice develop reduced microbial diversity, suggesting that the complement system may also regulate the skin microbiota.29 A mouse model of AD showed that application of a Vitreoscilla filiformis bacterial lysate reduced the inflammatory manifestations following allergen application.24 Studies in mice during the neonatal period suggest that tolerance to skin commensals such as S. epidermidis is preferentially established early in life. This supports the hypothesis that exposure to certain microbes at a critical window early in life is required for normal development of the immune system.30 5.2 Food allergy The potential role of the gut microbiome in food allergy has been studied in multiple murine models. Rodriguez et al92 demonstrated that intestinal colonization with Staphylococcus protects against oral sensitization and allergic responses. The microbiota of allergen‐sensitized IL‐4raF709 mice differentially promoted OVA‐specific IgE responses and anaphylaxis when reconstituted in wild‐type germ‐free mice, which could play a role in food allergy.93 The disease‐susceptible IL‐4raF709 mice display enhanced signalling through the interleukin‐4 receptor (IL‐4R) and exhibit STAT6‐dependent impaired generation and function of mucosal allergen‐specific Treg cells, which failed to suppress mast cell activation and expansion.94 Interestingly, STAT6 gene variants are also implicated in the pathophysiology of food allergy in humans.95 The gut microbiota can also regulate Th2 responses through the induction of RORγt Treg cells and Th17 cells.96 Certain bacterial strains such as Bifidobacterium longum 35624, Lactobacillus rhamnosus JB‐1, Clostridia species and Bacteroides fragilis can induce intestinal Treg cells that are able to suppress food allergy and colitis.97, 98 Pattern‐recognition receptor activation on DCs is a potential mechanism by which intestinal microbes may promote Treg cell differentiation.99 5.3 Asthma Important insights regarding the role of the microbiota in the pathogenesis of airway inflammation have come from mouse models. Neonatal mice are more susceptible to develop house dust mite (HDM)‐induced allergic airway inflammation (AAI) and airway hyper‐responsiveness (AHR) than mature mice.100 This phenomenon was associated with a shift from Gammaproteobacteria and Firmicutes towards a Bacteroidetes‐dominated microbiota and the development of PDL‐1–dependent Helios‐ Treg cells.100 Mice housed under germ‐free conditions display significantly more pronounced type 2 inflammation and AHR as compared to conventionally colonized mice. Recolonization, especially early in life, can reverse many of these immunological defects.101 Similarly, antibiotic‐driven dysbiosis in neonatal mice leads to impaired maturation of Tregs and enhanced Th2 responses and promotes proinflammatory colonic iNKT cells.80, 102-105 Conversely, specific bacterial strains, their components or metabolites can successfully induce a variety of anti‐inflammatory responses in the gut and in the lung. L. rhamnosus decreased AAI and AHR induced by Bet v 1 in mice.106 Bacterial strains isolated from neonatal mouse lungs and then administered intranasally very early in life (starting at day 2 after birth) can protect or worsen HDM‐induced airway inflammation, depending which cytokine profile they induced in vitro on precision‐cut lung slices.107 Intramuscular treatment with a DNA plasmid encoding a M. leprae 65 kDa heat‐shock protein (DNA‐HSP65) or subcutaneous injections with proteins from M. tuberculosis delivered in the presence of the TLR9 agonist CpG were able to significantly inhibit development of Der p 1‐induced AAI and AHR in MyD88‐ or Fas‐dependent manner.108 In addition, an exopolysaccharide from B. longum subsp. longum 35624 was shown to protect against colitis and AAI in murine models, which was dependent on TLR2‐induced IL‐10 secretion.109, 110 SCFAs or dietary fibres that are metabolized to SCFAs potently reduced experimental asthma, as well as increased the levels of colonic Bacteroidetes and Actinobacteria species, while decreasing the levels of Firmicutes and Proteobacteria.111, 112 Importantly, the beneficial effects of SCFAs or a high‐fibre diet were transferred to the offspring after treatment of pregnant mice via epigenetic mechanisms.112, 113 Mechanistically, SCFAs have been repeatedly shown to increase Treg numbers and effectiveness.114, 115 In addition, SCFAs influence bone marrow haematopoiesis,111 reduce effector T‐cell activity,116 improve epithelial barrier117, 118 and inhibit mast cell and ILC2 activation.119, 120 Other bacterial metabolites, such as histamine, can induce a wide and complex spectrum of regulatory mechanisms.121, 122 Increased numbers of histamine‐secreting bacteria were observed in adult patients with asthma and correlated with asthma severity.123 Histamine signalling through the H2R is involved in AAI,124 while the use of H2R antagonists in children during their first 6 months of life is associated with significantly increased risk of allergic diseases and asthma.10 6 THERAPEUTIC TARGETING OF THE MICROBIOME Despite the growing number of studies that associate changes in the microbiota with allergic and immune‐related outcomes, only a relatively small number of studies have shown clinical benefits and there are no microbe‐based therapies that are currently universally accepted for the prevention or treatment of allergies or asthma. A number of reasons can be suggested for this, which may include the poor choice of therapeutic microbes to begin with. It is likely that many confounding factors do influence the success of a microbiome therapeutic, such as diet, age, obesity, ethnicity and other environmental exposures. These need to be taken into account and controlled for. In addition, given the explosion in knowledge regarding disease endotypes, it is possible that specific microbes will need to be carefully selected to mechanistically fit with specific disease endotypes and it is likely that one intervention will not work for everyone. Certain interventions such as faecal transplantation may be too crude an approach, and until critical safety concerns are resolved, this type of intervention should not be considered outside the setting of carefully monitored clinical trials. 6.1 Atopic dermatitis Early intervention aimed at protecting the skin barrier may ameliorate progression of the atopic march in a subset of patients.19 Skin microbiome manipulation may offer novel therapeutic opportunities, as has been seen with the emollients supplemented with a Vitreoscilla filiformis lysate.125 Similarly, topically administration of Roseomonas mucosa improved clinical severity scores in adults and children with AD.125 Autologous microbiome transplant (AMT) of S. hominis and S. epidermidis showed efficacy in controlling S. aureus overgrowth.126 In addition to topical bacterial treatments, oral administration of probiotics has also been examined. Prenatal and post‐natal treatment with Lactobacillus and Bifidobacterium strains can reduce risk of AD development in infants,35, 127, 128 which may associate with changes in T cell–mediated responses.129 A mixture of probiotic strains was recently shown to reduce SCORAD index and topical steroid use in children with AD.130 Little has been reported on probiotic treatment of adults with AD, but administration of B. longum 35624 to adults with psoriasis resulted in reduced circulating CRP, TNF and IL‐17 levels, possibly due to increased numbers of Tregs, which suggests that bacteria in the gut can influence skin inflammatory activity in adults.131, 132 Taken together, supplementation with specific probiotic strains may modulate the gut bacteria in a way that influences inflammation within the skin and may protect some children against AD development.35 6.2 Food allergy The use of probiotics in food allergy treatment and prevention has been examined. Supplementation of cow's milk‐allergic children with Lactobacillus casei and Bifidobacterium lactis did not accelerate cow's milk allergy resolution.133 However, the combination of L. rhamnosus GG and extensively hydrolysed casein formula did accelerate milk allergy resolution after 6 and 12 months when compared to the formula‐only control group.134 The combination of L. rhamnosus supplementation and peanut oral immunotherapy (OIT) was evaluated in peanut‐allergic children for 18 months. The combination was effective in inducing possible sustained unresponsiveness and immune changes that suggested modulation of the peanut‐specific immune response.135 In addition, a sustained beneficial effect on psychosocial impact of food allergy at 3 and 12 months after end of treatment was recently reported.136 However, the major limitation of this study is that further work is required to determine the relative contributions of the probiotic vs OIT due to the lack of an OIT and L. rhamnosus supplementation control groups in this trial. 6.3 Asthma A significant number of studies have examined the effect of probiotic supplementation on asthma‐related outcomes. A recent systematic review of probiotic studies in children with asthma identified eleven studies eligible with a total of 910 children. The proportion of children with fewer episodes of asthma was significantly higher in the probiotic group than in the control group, but no statistical significance was observed in childhood asthma control test, asthmatic symptom in the day and night, the number of symptom‐free days, forced expiratory volume in the first second predicted and peak expiratory flow.137 In the future, it will be interesting to evaluate microbial administration directly to the airways, in addition to the gut.138 7 CONCLUSIONS Significant advances have been made in recent years in describing the composition of the microbiome in the gut, airways and skin. The changes in bacterial communities that associate with, or sometimes precede, atopic dermatitis, food allergy and asthma are being identified (summarized in Table 1). Accumulating evidence suggests that microbial exposures might be most effective at preventing atopic disorders during the first 1‐2 years of life. However, substantial gaps in our knowledge on the microbiome still exist. In particular, the field has been slow to translate potentially effective microbiome‐associated therapies into the clinic via appropriate clinical trials performed to high standards and showing meaningful clinical responses that are superior to current avoidance approaches. While the critical role of the microbiota in cancer immunotherapy has been established, there are currently no published data on the potential role of the microbiota in influencing the success of immunotherapy or biologics in allergy or asthma.139 In addition, novel probiotics and not just the traditional probiotic strains need to be clinically tested. Furthermore, microbial components or their metabolites should also be examined; in particular, the application of these novel microbial drugs to the diseased site (eg, the airways) must be explored. Lastly, there are no microbial therapeutics currently approved for routine clinical practice, and significant effort and investment are still required to identify the optimal microbial interventions for allergy and asthma. Location Phyll (Genus) Effect Reference Oral cavity ↑ Gemella haemolysans ↓ Lactobacillus gasseri, Lactobacillus crispatus Increased risk of allergic diseases 47 Intestine ↑ Staphylococcus species Protection against oral sensitization and allergic responses 92 Intestine ↑ Clostridia, Firmicutes Milk allergy resolution 49 Intestine ↑ Lachnospiraceae, Ruminococcaceae Associated with egg allergy 50 Intestine ↓ Haemophilus, Dialister, Dorea, Clostridium Associated with food sensitization 51 Intestine ↓ Citrobacter, Oscillospira, Lactococcus, Dorea Associated with food allergy 51 Intestine ↓ Escherichia coli High faecal calprotectin, impaired IL‐10 activation, increased risk of AD and asthma 43 Intestine ↓ Bifidobacterium Correlates with AD severity 37 Intestine Early colonization with C. difficile Associated with AD development 38 Intestine ↓ Bacteroidetes diversity Associated with AD development 39 Intestine ↓ Akkermansia muciniphila, Ruminococcus gnavus and Lachnospiraceae Associated with AD development 40 Intestine ↓ Lachnospira, Veillonella, Faecalibacterium, Rothia Reduced levels of faecal SCFAs, increased risk of asthma 56 Intestine ↓ Bifidobacterium, Akkermansia, Faecalibacterium ↑ Candida, Rhodotorula Increased risk of developing multisensitized atopy, increased circulating proinflammatory metabolites 58 Upper airways Early colonization with Staphylococcus species, Corynebacterium, Dolosigranulum, Moraxella Associated with lower rate of respiratory infections in the first 2 years of life 65-67 Upper airways Early colonization with Streptococcus, Moraxella, Haemophilus Increased risk of virus‐induced acute respiratory infections and increased risk of asthma 60 Upper airways ↑ Proteobacteria Associated with rhinitis in children 69 Nasopharynx ↑ Haemophilus influenzae, Streptococcus species Increased risk of hospitalization during RSV infection 62 Nasopharynx Colonization with Staphylococcus aureus Decreased risk of hospitalization during RSV infection 63 Nasopharynx ↑ Streptococcus, Staphylococcus Abnormalities in functional tests of the respiratory system 64 Nasopharynx ↑ Staphylococcus aureus ↓ P. acnes Associated with high IgE levels 87 Nasopharynx ↑ Firmicutes (Staphylococcus & Streptococcus), Proteobacteria (Haemophilus, Pseudomonas & Moraxella), Fusobacteria ↓ Corynebacterium Associated with CRS in adults 88 Nasopharynx ↑ Streptococcus, Haemophilus, Fusobacteria ↓ Diversity Associated with CRS without nasal polyps in adults 89 Nasopharynx ↑ Lactobacillus during acute respiratory infection with RSV Reduced risk of wheezing 59 Hypopharynx Colonization with Moraxella catarrhalis, Haemophilus influenzae, Streptococcus pneumoniae Low‐grade systemic inflammation 61 Lower airways ↑ Proteobacterium (Klebsiella species) (Mycoplasma pneumoniae, Chlamydophila pneumoniae) Associated with severe asthma 77, 79 Lower airways ↑ Actinobacteria Improvement in asthma control 77 Lower airways ↑ Neisseria, Haemophilus, Campylobacter, Leptotrichia Associated with resistance to corticosteroids in asthma 75 Sputum ↑ Proteobacteria Associated with neutrophilic asthma exacerbations 78 Sputum ↑ Bacteroidetes Associated with eosinophilic asthma exacerbations 78 Skin ↑ Staphylococcus aureus Epidermal barrier dysfunction, cutaneous inflammation, formation of AD skin lesions, associated with AD severity and allergen sensitization, associated with susceptibility to eczema herpeticum among AD patients 19, 21, 23 Skin Colonization with single clonal Staphylococcus aureus strains Associated with AD severity 26 Skin ↑ Malassezia species. Associated with AD severity 35 Skin ↑ Corynebacterium, Proteobacterium Associated with AD severity 21 Skin ↑ coagulase‐negative staphylococci: (Staphylococcus epidermidis, S. hominis, S. lugdunensis) Limits Staphylococcus aureus overgrowth 28 Skin Colonization with S. epidermidis TLR2 activation, epidermal barrier maintenance 1 Skin ↓ Proteobacteria (Propionibacterium, Streptococcus, Acinetobacter, Corynebacterium, Prevotella) Associated with AD 28, 30 Skin Early colonization with S. epidermidis Local activation of the host immune response through induction of S. epidermidis‐specific FOXP3 Treg cells 29 Skin ↑ in resident skin bacteria Associated with AD flares 24 This table summarizes the bacterial changes that have been associated with atopic dermatitis, food allergy or asthma. CONFLICTS OF INTEREST LOM is a consultant to Alimentary Health Ltd and has received research funding from GSK. NL, PS, ZL, MS and TE have no conflict of interest in relation to this work. AUTHOR CONTRIBUTIONS NL, PS, ZL, MS, TE and LOM contributed to drafting the manuscript. All authors read, reviewed and agreed the final version of this manuscript. REFERENCES Notes : This table summarizes the bacterial changes that have been associated with atopic dermatitis, food allergy or asthma.
Editorial from The New England Journal of Medicine — Oral Desensitization to Peanuts..
A series of case reports and small studies have shown that the systematic introduction of tiny amounts of peanut allergen, followed by gradual increases in dose, could prevent or attenuate systemic reactions.1-4 The concept gained traction when a group in Cambridge, United Kingdom, found that 12% defatted peanut flour could induce desensitization in children.5 Vickery and colleagues now present in the Journal6 the results of a randomized, controlled trial involving approximately 550 participants with peanut allergy. The trial used a Good Manufacturing Process–produced 12% defatted peanut flour preparation, known as AR101, as the allergen.
AR101 and other, similar products such as CA002, which is being developed by the Cambridge group, would therefore appear to have a role in initial dose escalation. The potential market for these products is believed to be billions of dollars.10 It is perhaps salutary to consider that in the study conducted by the Cambridge group, children underwent desensitization with a bag of peanut flour costing peanuts. . Via Krishan Maggon
1 Background There are limited data on the feasibility, efficacy and safety of high‐dose oral immunotherapy (OIT) in children highly allergic to peanuts. 2 Objective In children highly allergic to peanut, we primarily aimed to determine the feasibility of reaching the maximum maintenance dose (MMD) of 5000 mg peanut protein or, alternatively, a lower individual maintenance dose (IMD), by OIT up‐dosing. Secondarily, we aimed to identify adverse events (AEs) and determine factors associated with reaching a maintenance dose. 3 Methods The TAKE‐AWAY peanut OIT trial enrolled 77 children 5‐15 years old, with a positive oral peanut challenge. Fifty‐seven were randomized to OIT with biweekly dose step‐up until reaching MMD or IMD and 20 to observation only. Demographic and biological characteristics, AEs, medication and protocol deviations were explored for associations with reaching maintenance dose. 4 Results All children had anaphylaxis defined by objective symptoms in minimum two organ systems during baseline challenge. The MMD was reached by 21.1%, while 54.4% reached an IMD of median (minimum, maximum) 2700 (250, 4000) mg peanut protein, whereas 24.5% discontinued OIT. During up‐dosing, 19.4% experienced anaphylaxis. Not reaching the MMD was caused by distaste for peanuts (66.7%), unacceptable AEs (26.7%) and social reasons (6.7%). Increased peanut s‐IgG4/s‐IgE ratio (OR [95% CI]: 1.02 [1.00, 1.04]) was associated with reaching MMD. 5 Conclusion Although 75.5% of children with peanut anaphylaxis reached a maintenance dose of 0.25‐5 g, only 21.1% reached the MMD. Distaste for peanuts and AEs, including high risk of anaphylaxis, limited the feasibility of reaching MMD. Via Krishan Maggon
PEANUT ALLERGY ACCOUNTS FOR THE MAJORITY of severe food-related allergic reactions. It tends to present early in life, and affected individuals generally do not outgrow it. In highly sensitized people, trace quantities can induce an allergic reaction.
Scientists says blood test could avoid costly, stressful, food tests for confirming allergy
Aimmune Therapeutics develops treatments to protect children with food allergies (peanut, milk, and egg) from the consequences of accidental exposure. Via Krishan Maggon
Adopting the new peanut guidelines may be difficult for doctors and parents and will require further education.
Antigen-specific immunotherapy (AIT) is a promising therapeutic approach for both cow’s milk allergy (CMA) and peanut allergy (PNA), but needs optimization in terms of efficacy and safety. Compare oral immunotherapy (OIT) and subcutaneous immunotherapy (SCIT) in murine models for CMA and PNA and determine the dose of allergen needed to effectively modify parameters of allergy. Female C3H/HeOuJ mice were sensitized intragastrically (i.g.) to whey or peanut extract with cholera toxin. Mice were treated orally (5 times/week) or subcutaneously (3 times/week) for three consecutive weeks. Hereafter, the acute allergic skin response, anaphylactic shock symptoms and body temperature were measured upon intradermal (i.d.) and intraperitoneal (i.p.) challenge, and mast cell degranulation was measured upon i.g. challenge. Allergen-specific IgE, IgG1 and IgG2a were measured in serum at different time points. Single cell suspensions derived from lymph organs were stimulated with allergen to induce cytokine production and T cell phenotypes were assessed using flow cytometry. Both OIT and SCIT decreased clinically related signs upon challenge in the CMA and PNA model. Interestingly, a rise in allergen-specific IgE was observed during immunotherapy, hereafter, treated mice were protected against the increase in IgE caused by allergen challenge. Allergen-specific IgG1 and IgG2a increased due to both types of AIT. In the CMA model, SCIT and OIT reduced the percentage of activated Th2 cells and increased the percentage of activated Th1 cells in the spleen. OIT increased the percentage of regulatory T cells (Tregs) and activated Th2 cells in the MLN. Th2 cytokines IL-5, IL-13 and IL-10 were reduced after OIT, but not after SCIT. In the PNA model, no differences were observed in percentages of T cell subsets. SCIT induced Th2 cytokines IL-5 and IL-10, whereas OIT had no effect. We have shown clinical protection against allergic manifestations after OIT and SCIT in a CMA and PNA model. Although similar allergen-specific antibody patterns were observed, differences in T cell and cytokine responses were shown. Whether these findings are related to a different mechanism of AIT in CMA and PNA needs to be elucidated. |