Follow, research and publish the best content
Get Started for FREE
Sign up with Facebook Sign up with X
I don't have a Facebook or a X account
Already have an account: Login
 Your new post is loading... Your new post is loading...
 Your new post is loading... Your new post is loading...
|




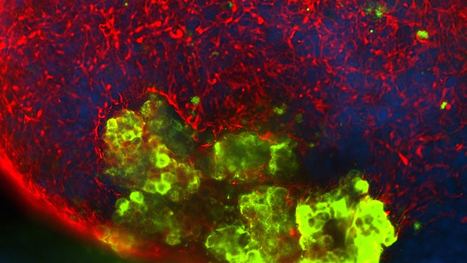






The rapid development of pig genetic engineering, particularly with the introduction of genome editing techniques such as CRISPR-Cas, has accelerated the generation of new pig lines with multiple modifications. With pre-clinical testing in progress, it is an opportune time to consider any implications of genetic modification for the conditions for undertaking clinical trials. The assessment of clinical trial protocols using GM pig islets will need to be performed on a case-by-case basis, taking into account a range of factors including the particular genetic modification(s) and the site and method of delivery.