Mass spectrometry was used to investigate the role of PpKAI2L protein in the moss Physicomitrium patens as receptor of a class of phytohormones , striptolactones, in the moss Physcomitrium patens and to compare this processes with the more known vascular plants. Results on Physcomitrium highlight surprising evolutive innovations from vascular plant.
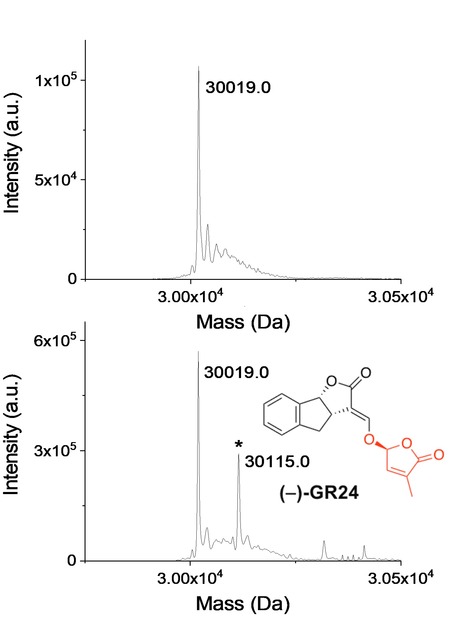
Abstract from The Plant Clell article: Strigolactones (SL) make up a novel class of phytohormones that are found across the whole land plant lineage. In vascular plants, the main hormonal role of SL is the repression of shoot axillary branching. However, SL are also a major symbiotic signal, granting the plant increased access to the nutrients and water contained in the rhizosphere. These two functions of SL led to the hypothesis that these molecules have been instrumental at the time of land colonization by plants, approximately 450 million years ago. Studying SL biosynthesis and signaling in the bryophyte Physcomitrium patens (P. patens, a non-vascular plant), and comparing these processes with the available knowledge in vascular plants, enables to investigate the evolution of SL cellular pathways in land plants. In angiosperms, the perception of SLs relies on a receptor called D14 (encoded by the same gene family as KAI2) along with the F-box protein MAX2. In moss, Max2 is not required for the SL response although it possesses 13 KAI2-like genes (PpKAI2L). An unusual aspect of SL perception is that the D14 protein is both a receptor and an enzyme that cleaves its substrate (and covalently binds part of the SL) in a signaling mechanism that is still under debate. To further investigate whether PpKAI2L proteins play roles as receptors of SLs and related compounds, we examined the covalent attachment of the artificial SL GR24 isomers to the PpKAI2L proteins by mass spectrometry (MS). Analyses revealed 96 Da increments (corresponding to the D ring mass) when AtKAI2 and PpKAI2L were incubated with GR24 isomers indicating that moss PpKAI2L proteins, like vascular plant receptors, covalently link GR24 enantiomers. As SL signaling is not conserved in P. patens, it appears that the known SL signaling pathway results from a vascular plants specific innovation. Likewise, SL response in P. patens would be the product of a convergent evolution. Therefore, the question as to how P. patens transduces the SL signal, downstream of perception by specific PpKAI2L proteins, remains open. This work was conducted by a team of INRAE (Sandrine Bonhomme) in collaboration with other teams of french institute (ICSN) and laboratory (LBPV). Mass spectrometry analyses were performed at the I2BC proteomics platform.
More information here.
Contact person: David Cornu




 Your new post is loading...
Your new post is loading...