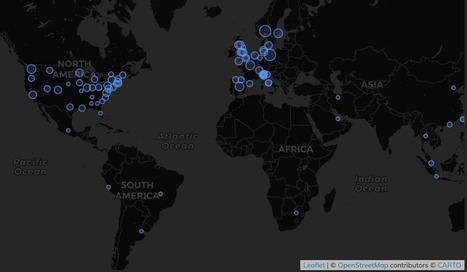
Given the accelerated rate at which trial information and findings are emerging, an urgent need exists to track clinical trials, avoid unnecessary duplication of efforts, and understand what trials are being done and where. In response, we have developed a COVID-19 clinical trials registry to collate all trials.
Data are pulled from the International Clinical Trials Registry Platform, including those from the Chinese Clinical Trial Registry, ClinicalTrials.gov, Clinical Research Information Service - Republic of Korea, EU Clinical Trials Register, ISRCTN, Iranian Registry of Clinical Trials, Japan Primary Registries Network, and German Clinical Trials Register. Both automated and manual searches are done to ensure minimisation of duplicated entries and for appropriateness to the research questions. Identified studies are then manually reviewed by two separate reviewers before being entered into the registry.
Concurrently, we have developed artificial intelligence (AI)-based methods for data searches to identify potential clinical studies not captured in trial registries. These methods provide estimates of the likelihood of importance of a study being included in our database, such that the study can then be reviewed manually for inclusion. Use of AI-based methods saves 50–80% of the time required to manually review all entries without loss of accuracy. Finally, we will use content aggregator services, such as LitCovid, to ensure our data acquisition strategy is complete. With this three-step process, the probability of missing important publications is greatly diminished and so the resulting data are representative of global COVID-19 research efforts.
Published in The Lancet (April 24, 2020)
https://doi.org/10.1016/S2589-7500(20)30086-8
Clinical Trial Registry Site:
Via Juan Lama


 Your new post is loading...
Your new post is loading...