The rapid identification of a correlate of protection (CoP) for Covid-19 vaccines — on the basis of several harmonized randomized phase 3 trials using common validated assays — constitutes an important success in vaccinology. A CoP is an immune marker that can be used to reliably predict a vaccine’s level of efficacy in preventing a clinically relevant outcome. The level of this marker is measured shortly (2 to 4 weeks) after completion of the vaccination regimen and provides an actionable basis for decisions such as regulatory approval of an efficacious vaccine for a new population that was not included in the pivotal randomized phase 3 trials, or approval of a refined version of a vaccine that was previously shown to be efficacious. Once established, a CoP can be used as the primary end point for provisional or full approval of a vaccine for a specific use, if a clinical immunobridging study confirms that high enough levels of the CoP are achieved. For example, the Food and Drug Administration (FDA) extended approval of the mRNA-1273 (Moderna) and BNT162b2 (Pfizer–BioNTech) Covid vaccines from older to younger age groups on the basis of a comparison of neutralizing antibody titers. Moreover, FDA guidance and a European Medicines Agency declaration from the International Coalition of Medicines Regulatory Authorities recommended that approval of new vaccine strains and booster doses be based on clinical immunobridging studies showing noninferiority or superiority with respect to a CoP end point. Other applications of a CoP include ensuring vaccine consistency from lot to lot, supporting recommendations for coadministration with other vaccines, and determination of appropriate expiration dates.
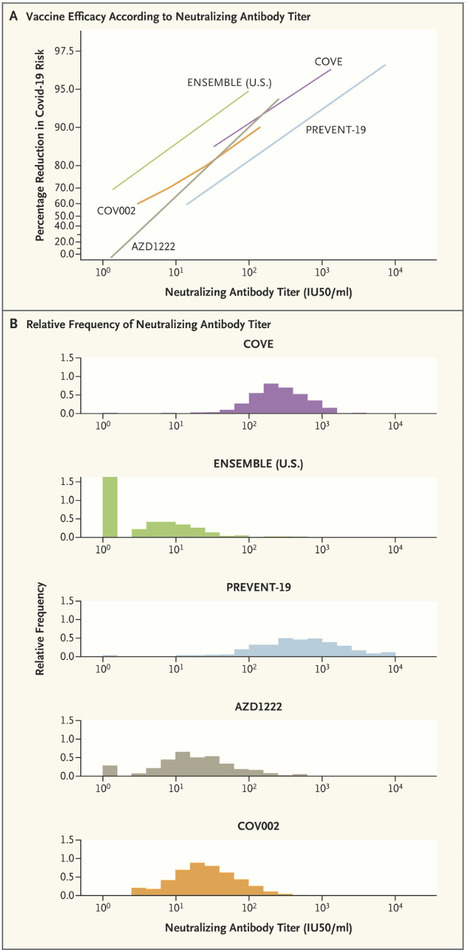
Confusion about CoPs is understandable, given the myriad complicated issues involved in identifying them and the fact that different uses for CoPs require different validation measures. Evidence that a marker is a CoP is generally derived from five main sources: natural history studies that correlate infection-induced immune responses with outcomes; vaccine-challenge studies in animals or humans; studies that experimentally manipulate the immune marker to directly assess mechanistic causation (e.g., by administering various vaccine doses or using passive antibody transfer); efficacy trials that quantify the relationship between vaccine efficacy and the level of the immune marker in individual vaccine recipients; and meta-analyses of series of efficacy trials that correlate vaccine efficacy with the mean immune-marker level. Strong evidence has been generated from all five of these sources for both serum anti-spike IgG concentration and anti–SARS-CoV-2 neutralizing antibody titer as CoPs for vaccines against symptomatic Covid-19; for brevity, we focus here on the neutralizing antibody titer. Meta-analyses have established high correlations between the standardized mean titer and vaccine efficacy, and the neutralizing antibody titer has consistently been shown to be a mechanistic CoP in challenge studies in nonhuman primates. The U.S. government’s COVID-19 Vaccine Correlates of Protection Program assessed CoPs in phase 3 trials of four vaccines: COVE for mRNA-1273, ENSEMBLE for Ad26.COV2.S, PREVENT-19 for NVX-CoV2373, AZD1222 (United States/Chile/Peru) for ChAdOx1 nCoV-19, and COV002 (United Kingdom) also for ChAdOx1 nCoV-19. Vaccine efficacy always markedly increased with the titer.
Published in NEJM (Dec. 10, 2022):
https://doi.org/10.1056/NEJMp2211314
Via Juan Lama


 Your new post is loading...
Your new post is loading...