 Your new post is loading...
Researchers have developed a biosynthetic “clock” that keeps cells from reaching normal levels of deterioration related to aging. They engineered a gene oscillator that switches between the two normal paths of aging, slowing cell degeneration and setting a record for life extension. Human lifespan is related to the aging of our individual cells. Three years ago a group of University of California San Diego researchers deciphered essential mechanisms behind the aging process. After identifying two distinct directions that cells follow during aging, the researchers genetically manipulated these processes to extend the lifespan of cells. As described April 28, 2023 in Science, they have now extended this research using synthetic biology to engineer a solution that keeps cells from reaching their normal levels of deterioration associated with aging. Cells, including those of yeast, plants, animals and humans, all contain gene regulatory circuits that are responsible for many physiological functions, including aging. “These gene circuits can operate like our home electric circuits that control devices like appliances and automobiles,” said Professor Nan Hao of the School of Biological Sciences’ Department of Molecular Biology, the senior author of the study and co-director of UC San Diego’s Synthetic Biology Institute. However, the UC San Diego group uncovered that, under the control of a central gene regulatory circuit, cells don’t necessarily age the same way. Imagine a car that ages either as the engine deteriorates or as the transmission wears out, but not both at the same time. The UC San Diego team envisioned a “smart aging process” that extends cellular longevity by cycling deterioration from one aging mechanism to another. In this new study, the researchers genetically rewired the circuit that controls cell aging. From its normal role functioning like a toggle switch, they engineered a negative feedback loop to stall the aging process. The rewired circuit operates as a clock-like device, called a gene oscillator, that drives the cell to periodically switch between two detrimental “aged” states, avoiding prolonged commitment to either, and thereby slowing the cell’s degeneration. These advances resulted in a dramatically extended cellular lifespan, setting a new record for life extension through genetic and chemical interventions. As electrical engineers often do, the researchers in this study first used computer simulations of how the core aging circuit operates. This helped them design and test ideas before building or modifying the circuit in the cell. This approach has advantages in saving time and resources to identify effective pro-longevity strategies, compared to more traditional genetic strategies. “This is the first time computationally guided synthetic biology and engineering principles were used to rationally redesign gene circuits and reprogram the aging process to effectively promote longevity,” said Hao. Several years ago the multidisciplinary UC San Diego research team began studying the mechanisms behind cell aging, a complex biological process that underlies human longevity and many diseases. They discovered that cells follow a cascade of molecular changes through their entire lifespan until they eventually degenerate and die. But they noticed that cells of the same genetic material and within the same environment can travel along distinct aging routes. About half of the cells age through a gradual decline in the stability of DNA, where genetic information is stored. The other half ages along a path tied to the decline of mitochondria, the energy production units of cells. The new synthetic biology achievement has the potential to reconfigure scientific approaches to age delay. Distinct from numerous chemical and genetic attempts to force cells into artificial states of “youth,” the new research provides evidence that slowing the ticks of the aging clock is possible by actively preventing cells from committing to a pre-destined path of decline and death, and the clock-like gene oscillators could be a universal system to achieve that.
Aber-clam Lincoln, a quahog clam believed to be 214 years old found at Alligator Point, was released into the Gulf of Mexico Friday by his caretakers at the Gulf Specimen Marine Lab in Panacea. Americorps member Blaine Parker dug up the two-century old mollusk while collecting shellfish to make chowder. Parker said it is hefty enough to make two servings and has shells large enough to use as bowls to serve it in. “We were just going to eat it, but we thought about it a while and figured it was probably pretty special. So, we didn’t want to kill it,” said Parker. Instead, he took it to the aquarium at the Gulf Specimen Marine Lab where he works as a specimen collector. While most Ocean quahogs are within 2.8 to 4.3 inches in length and weigh up to a half pound, Lincoln checks in at 6 inches and weighs 2.6 pounds. The clam has been drawing up to 100 visitors a day. Parker is a recent Eckerd College graduate with a degree in environmental studies and marine science. He said, a clam, like a tree, lays down annual growth bands, alternating bands of light on its shell, that scientists used to estimate its age. There are 214 layers on the shell of the clam he found, which would mean it was likely born in 1809, the same year as Abraham Lincoln. It was also discovered on Presidents Day weekend. So, Parker named his find Aber-clam Lincoln. Quahogs occur in the Atlantic from Greenland south to North Carolina. They move about by being tossed in the surf, and crawl along the sand by expanding and contracting their muscle. With Lincoln being so old it is possible, like many Floridians, circumstances moved him south for him to become one of the oldest known Florida transplants. Quahogs are known to live 200 or more years. A 507-year-old clam, found in the Icelandic seabed and named Ming, broke the Guinness World Record for oldest animal when it was discovered in 2007. Scientists think an incredible low metabolic rates of Lincoln and other quahogs is responsible for their long life. A University of Kansas study of the energy needs of 299 species of extinct and living bivalves found those with minimal energy requirements escaped extinction.
Telomeres are "caps" of non-coding DNA sequences present at both tips of all chromosomes, which are extremely important in the aging process. These caps protect DNA as cells go through various life cycles of replication, however, each time a cell divides these telomeres are shortened and eventually contribute to disease and cellular aging. Now, exciting new research published in the Journal PNAS has shown that an experimental gene therapy could be used to halt the shortening of these telomere caps in mice and by doing so increase the life span of these animals by up to 41 percent compared to controls. Telomere length can be considered a marker of biological age and its shortening is a hallmark of a process called cellular senescence, which limits the replication of DNA in old damaged cells. As we age, telomere caps become shorter and shorter until the cell's DNA becomes vulnerable to damage by cellular stresses that could lead to diseases such as cancer. Or the cell could ultimately reach senescence where it will no longer be able to replicate and so contribute to the aging process. For scientists looking at how to slow or even reverse aging, telomeres are of great interest. One of the reasons telomeres shorten over time is due to the reduced activity of an enzyme called telomerase which is responsible for maintaining the length of the telomere caps on chromosomes. The function of telomerase relies on a complex called telomerase reverse transcriptase (TERT). This complex activates telomerase, allowing the enzyme to be biologically active in cells, which in turn lengthens the telomeres on the tips of chromosomes. The authors of the new study developed a gene therapy protocol using cytomegaloviruses (CMV) to deliver TERT to cells in different organs of mice. They wanted to see if it could increase telomerase activity responsible for lengthening telomere tips in biologically older animals versus those given a mock delivery as a control group. They then compare the results to normal wild-type animals of a similar biological age. The scientists were able to successfully deliver the gene to the animals using their protocol. They found the telomeres of chromosomes in some organs, such as the kidney, increased in length in TERT treated mice vs controls and that it had an overall positive effect on glucose metabolism which is known to decline during aging. The results also showed evidence that the treatment helped to prevent hair loss and improved the animal's motor activity and coordination. Moreover, the scientists say that the treatment with TERT did not increase the cancer risk of these animals, which is normally a concern in telomere research. Overall, TERT-treated animals had an extended lifespan of 41 percent compared to mock and wild-type control animals, which is a promising result. Alongside TERT delivery, the authors used the same protocol with a gene for follistatin in a different cohort of mice. Follistatin is involved in maintaining skeletal muscle development, which is also known to decline as we age. The animals that received follistatin also had an increased lifespan of 32 percent, with similar benefits to those seen in the TERT-treated mice, illustrating both TERT and Follistatin may be able to offset biological aging in mouse models. "We report CMV being used successfully as both an intranasal and injectable gene therapy system to extend longevity," the authors write. "Specifically, this treatment significantly improved glucose tolerance, physical performance, as well as preventing body mass loss and alopecia. Further, telomere shortening associated with aging was ameliorated by TERT and mitochondrial structure deterioration was halted in both treatments. Intranasal and injectable preparations performed equally well in safely and efficiently delivering gene therapy to multiple organs, with long-lasting benefits and without carcinogenicity or unwanted side effects." Going forward the scientists say that translational studies will be required to determine whether these results could be replicated in human subjects.
As the global elderly population grows, it is socioeconomically and medically critical to provide diverse and effective means of mitigating the impact of aging on human health. Previous studies showed that the adeno-associated virus (AAV) vector induced overexpression of certain proteins, which can suppress or reverse the effects of aging in animal models. In a recent study, a research team sought to determine whether the high-capacity cytomegalovirus vector (CMV) can be an effective and safe gene delivery method for two such protective factors: telomerase reverse transcriptase (TERT) and follistatin (FST). They found that the mouse cytomegalovirus (MCMV) carrying exogenous TERT or FST (MCMVTERT or MCMVFST) extended median lifespan by 41.4% and 32.5%, respectively. CMV was used successfully as both an intranasal and injectable gene therapy system to extend longevity. Specifically, this treatment significantly improved glucose tolerance, physical performance, as well as preventing body mass loss and alopecia. Further, telomere shortening associated with aging was ameliorated by TERT and mitochondrial structure deterioration was halted in both treatments. Intranasal and injectable preparations performed equally well in safely and efficiently delivering gene therapy to multiple organs, with long-lasting benefits and without carcinogenicity or unwanted side effects. Translating this research to humans could have significant benefits associated with quality of life and an increased health span. About the study In the present study, researchers determined the impact of a high-capacity cytomegalovirus vector (CMV) on factors like follistatin (FST) and telomerase reverse transcriptase (TERT) that protect against the influence of aging on human health. The team developed a mouse CMV (MCMV) vector that expressed luciferase as a reporter gene (MCMVLuc) in order to surveil MCMV infection and its replication in culture as well as in a mouse model. Replicated MCMVLuc along with its parental virus was used in this study as a wild-type MCMV virus inoculation (WT) or viral vector control. The team also generated recombinant MCMV vectors that expressed FLAG-tagged genes telomerase reverse transcriptase (TERT) and FST (MCMVTERT and MCMVFST). Furthermore, the team fused the mouse TERT with the FLAG-tag at the C-terminus using two intermediate amino acids, namely arginine and threonine. The expression kinetics of the target protein and the therapeutic effectiveness of CMV was determined by evaluating the levels of TERT in the blood samples of eight-month-old mice over a span of a month post-treatment. The extension of life after treatment was evaluated by administering seven out of nine groups of aged female mice with mock treatment intraperitoneally (IP), WT-treated intranasally (IN) (WT-IN), WT-IP, MCMVTERT-IN, MCMVTERT-IP, MCMVFST-IN, and MCMVFST-IP, respectively. The treatment was first started in 18-month-old mice. The team sacrificed one mouse in each group at 24 months to perform tissue analysis while the rest of the mice were monitored for changes in their physiology until their natural death. Furthermore, the length of telomeres in kidneys and muscle tissues was determined, and comparisons were drawn between those in different mice groups using quantitative fluorescence in situ hybridization (Q-FISH). The team also estimated the telomere length observed in the skeletal muscles of mice treated with TERT. The telomere length of various organs was also evaluated using real-time quantitative polymerase chain reaction (qPCR). Results The study results showed that the FLAG-tagged genes MCMVTERT and MCMVFST replicated to a degree comparable to that of MCMVLuc (WT) in the fibroblast cells in mice and in vivo. The expression of TERT protein when administered IN or IP was its highest seven days after treatment before gradually reducing to its basal level on day 25. This indicated that the vector was able to deliver proteins in an in vivo environment. Analysis of FST and TERT messenger ribonucleic acids (mRNAs) and proteins in blood and tissue samples obtained from treated animals. Analysis of the impact of treatment on life extension showed that the WT and mock control mice succumbed after a median duration of 26.7, 26.5, and 26.4 months. The median age of death in the MCMVFST-treated and the MCMVTERT-treated mice was 35.1 and 37.5 months, respectively. The team noted that the longest lifespan was observed in MCMVTERT-treated mice at 41.2 months, while that in the MCMVFST-treated mice was 38.0 months. The team remarked that CMV therapy was comparable in effectiveness irrespective of the route of inoculation, which suggested that the expression of genes was not significantly impacted by the interaction of the vector with the immune system. The results of the Q-FISH method showed an increase of 3.1-fold in the length of telomeres in the kidneys of TERT-treated mice in comparison to the untreated mice controls. Moreover, the number of telomeres observed in the kidneys of mice treated with TERT was significantly higher than those in the untreated mice or those treated with WT. Notably, no substantial changes were found in FST-treated or the WT-treated subjects which suggested the efficiency of TERT in the TERT-treated mice. The team also observed that the telomere length in the muscular tissues of the TERT-treated mice increased by almost three times as compared to those in the untreated subjects. In contrast, there was no substantial increase in the telomere length in the FST- or the WT-treated mice. Moreover, qPCR showed no variation in the length of the telomeres in the different tissues of mice treated with TERT. The team noted that the relative telomere length in the brain, heart, lung, liver, and kidney in 24-month-old MCMVTERT-treated mice was almost three times more than that in untreated mice of the same age. Conclusion Overall, the study findings showed the importance of investigating the impact of CMV TERT and FST vector usage against conditions related to aging, such as chronic inflammatory conditions, sarcopenia, type 2 diabetes, and heart diseases. Cited research published in P.N.A.S. (May 10, 2022): https://doi.org/10.1073/pnas.2121499119
Via Juan Lama
Over the last few decades, life expectancy has increased dramatically around the globe. The average person born in 1960, the earliest year the United Nations began keeping global data, could expect to live to 52.5 years of age. Today, the average is 72. In the UK, where records have been kept longer, this trend is even greater. In 1841, a baby girl was expected to live to just 42 years of age, a boy to 40. In 2016, a baby girl could expect to reach 83; a boy, 79. The natural conclusion is that both the miracles of modern medicine and public health initiatives have helped us live longer than ever before – so much so that we may, in fact, be running out of innovations to extend life further. In September 2018, the Office for National Statistics confirmed that, in the UK at least, life expectancy has stopped increasing. Beyond the UK, these gains are slowing worldwide. This belief that our species may have reached the peak of longevity is also reinforced by some myths about our ancestors: it’s common belief that ancient Greeks or Romans would have been flabbergasted to see anyone above the age of 50 or 60, for example. In fact, while medical advancements have improved many aspects of healthcare, the assumption that human life span has increased dramatically over centuries or millennia is misleading. Overall life expectancy, which is the statistic reflected in reports like those above, hasn’t increased so much because we’re living far longer than we used to as a species. It’s increased because more of us, as individuals, are making it that far.
“There is a basic distinction between life expectancy and life span,” says Stanford University historian Walter Scheidel, a leading scholar of ancient Roman demography. “The life span of humans – opposed to life expectancy, which is a statistical construct – hasn’t really changed much at all, as far as I can tell.”
Life expectancy is an average. If you have two children, and one dies before their first birthday but the other lives to the age of 70, their average life expectancy is 35. That’s mathematically correct – and it certainly tells us something about the circumstances in which the children were raised. But it doesn’t give us the full picture. It also becomes especially problematic when looking at eras, or in regions, where there are high levels of infant mortality. Most of human history has been blighted by poor survival rates among children, and that continues in various countries today. This averaging-out, however, is why it’s commonly said that ancient Greeks and Romans, for example, lived to just 30 or 35. But was that really the case for people who survived the fragile period of childhood, and did it mean that a 35-year-old was truly considered ‘old’? If one’s thirties were a decrepit old age, ancient writers and politicians don’t seem to have got the message. In the early 7th Century BC, the Greek poet Hesiod wrote that a man should marry “when you are not much less than 30, and not much more”. Meanwhile, ancient Rome’s ‘cursus honorum’ – the sequence of political offices that an ambitious young man would undertake – didn’t even allow a young man to stand for his first office, that of quaestor, until the age of 30 (under Emperor Augustus, this was later lowered to 25; Augustus himself died at 75). To be consul, you had to be 43 – eight years older than the US’s minimum age limit of 35 to hold a presidency. In the 1st Century, Pliny devoted an entire chapter of The Natural History to people who lived longest. Among them he lists the consul M Valerius Corvinos (100 years), Cicero’s wife Terentia (103), a woman named Clodia (115 – and who had 15 children along the way), and the actress Lucceia who performed on stage at 100 years old.
A study led by Fernando Colchero, University of Southern Denmark and Susan Alberts, Duke University, North Carolina, that included researchers from 42 institutions across 14 countries, provides new insights into the aging theory "the invariant rate of aging hypothesis," which states that every species has a relatively fixed rate of aging. "Human death is inevitable. No matter how many vitamins we take, how healthy our environment is or how much we exercise, we will eventually age and die," said Fernando Colchero. He is an expert in applying statistics and mathematics to population biology and an associate professor at Department of Mathematics and Computer Science, University of Southern Denmark. "We were able to shed light on the invariant rate of aging hypothesis by combining an unprecedented wealth of data and comparing births and deaths patterns on nine human populations with information from 30 non-human primate populations, including gorillas, chimpanzees and baboons living in the wild and in zoos," said Fernando Colchero. In order to explore this hypothesis, the researchers analyzed the relationship between life expectancy, this is the average age at which individuals die in a population, and lifespan equality, which measures how concentrated deaths are around older ages. Their results show that, as life expectancy increases, so does lifespan equality. So, lifespan equality is very high when most of the individuals in a population tend to die at around the same age such as observed in modern Japan or Sweden -- which is around their 70s or 80s. However, in the 1800s lifespan equality was very low in those same countries, since deaths were less concentrated at old ages, resulting also in lower life expectancy. "Life expectancy has increased dramatically and still does in many parts of the world. But this is not because we have slowed our rate of aging; the reason is that more and more infants, children and young people survive and this brings up the average life expectancy," said Fernando Colchero.
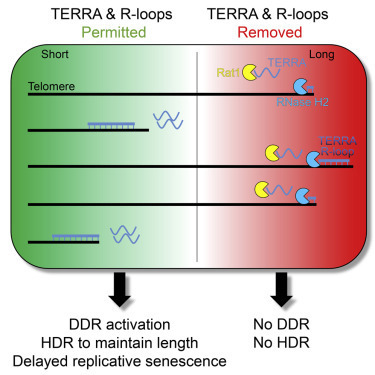
A new study by EPFL researchers shows how RNA species called TERRA muster at the tip of chromosomes, where they help to prevent telomere shortening and premature cell aging. Molecules that accumulate at the tip of chromosomes are known to play a key role in preventing damage to our DNA. Now, researchers at EPFL have unraveled how these molecules home in on specific sections of chromosomes—a finding that could help to better understand the processes that regulate cell survival in aging and cancer. Much like an aglet of a shoelace prevents the end of the lace from fraying, stretches of DNA called telomeres form protective caps at the ends of chromosomes. But as cells divide, telomeres become shorter, making the protective cap less effective. Once telomeres get too short, the cell stops dividing. Telomere shortening and malfunction have been linked to cell aging and age-related diseases, including cancer. Scientists have known that RNA species called TERRA help to regulate the length and function of telomeres. Discovered in 2007 by postdoc Claus Azzalin in the team of EPFL Professor Joachim Lingner, TERRA belongs to a class of molecules called noncoding RNAs, which are not translated into proteins but function as structural components of chromosomes. TERRA accumulates at chromosome ends, signaling that telomeres should be elongated or repaired. However, it was unclear how TERRA got to the tip of chromosomes and remained there. “The telomere makes up only a tiny bit of the total chromosomal DNA, so the question is ‘how does this RNA find its home?’”, Lingner says. To address this question, postdoc Marianna Feretzaki and others in the teams of Joachim Lingner at EPFL and Lumir Krejci at Masaryk University set out to analyze the mechanism through which TERRA accumulates at telomeres, as well as the proteins involved in this process. The findings are published in Nature.
There are a handful of people left on Earth who have been alive in three separate centuries, says Rachel Nuwer. What can they – and those of a similarly extreme age – teach us? Reaching a hundredth birthday is always cause for celebration, but these days there are so many centenarians around that scientists don’t even bother trying to keep track of them all. Indeed, in 2012 the United Nations estimated that there were about 316,600 people over 100 living around the world. By 2050, that number – unbelievably – is expected to rise to over three million. A much more exclusive club, therefore, are the supercentenarians, or people who live to 110 or older. The Gerontology Research Group, a global team headquartered in Los Angeles, maintains the go-to database for keeping track of the oldest among us. Until this week, there were 53 supercentenarians. Sadly, the death of the oldest, Misao Okawa of Japan, was announced on 1 April. She was 117. Okawa was born in 1898, and there are now just four living people – three Americans and one Italian, all women – who were born before 1900. That is, they have lived to see three centuries. You might call these four people “tricenta-centenarians*” if giving their group a name (although language experts may have a better suggestion), and what makes them unique is that the world won’t see another set until 2100. This loss will likely happen in less than a decade, as supercentenarians tend to hold their venerated title only fleetingly. Time’s unrelenting march means there’s a steady turnover of the world’s oldest, causing experts across many fields – biology, history, cultural anthropology – to scramble to learn what they can from these extraordinary people while they are still here. And it’s not only their health secrets that they stand to teach us. The most obvious reason to study the oldest people alive today is for clues to healthy ageing. Supercentenarians often “seem to be born with slower clocks than the rest of us,” says Stuart Kim, a developmental biologist at Stanford University. When supercentenarians are 60, they appear to be 40; when they are 90, they seem about 70. “When you meet them,” Kim says, “they all look and act 20 years younger than they actually are.”
New study suggests that plasma exchange could be the key to unlocking the body’s regenerative capacities. In 2005, University of California, Berkeley, researchers made the surprising discovery that making conjoined twins out of young and old mice — such that they share blood and organs — can rejuvenate tissues and reverse the signs of aging in the old mice. The finding sparked a flurry of research into whether a youngster’s blood might contain special proteins or molecules that could serve as a “fountain of youth” for mice and humans alike. But a new study by the same team shows that similar age-reversing effects can be achieved by simply diluting the blood plasma of old mice — no young blood needed. In the new study, the team found that replacing half of the blood plasma of old mice with a mixture of saline and albumin — where the albumin simply replaces protein that was lost when the original blood plasma was removed — has the same or stronger rejuvenation effects on the brain, liver and muscle than pairing with young mice or young blood exchange. Performing the same procedure on young mice had no detrimental effects on their health. This discovery shifts the dominant model of rejuvenation away from young blood and toward the benefits of removing age-elevated, and potentially harmful, factors in old blood. “There are two main interpretations of our original experiments: The first is that, in the mouse joining experiments, rejuvenation was due to young blood and young proteins or factors that become diminished with aging, but an equally possible alternative is that, with age, you have an elevation of certain proteins in the blood that become detrimental, and these were removed or neutralized by the young partners,” said Irina Conboy, a professor of bioengineering at UC Berkeley who is the first author of the 2005 mouse-joining paper and senior author of the new study . “As our science shows, the second interpretation turns out to be correct. Young blood or factors are not needed for the rejuvenating effect; dilution of old blood is sufficient.”
The roundworm Caenorhabditis elegans is helping scientists understand how the brain controls aging, including age-related muscle atrophy. While many of us worry about proteins aggregating in our brains as we age and potentially causing Alzheimer’s disease or other types of neurodegeneration, we may not realize that some of the same proteins are aggregating in our muscles, setting us up for muscle atrophy in old age. University of California, Berkeley, scientists have now found brain cells that help clean up these tangles and prolong life — at least in worms and possibly mice. This could lead to drugs that improve muscle health or extend a healthy human lifespan. The research team’s most recent discovery, published Jan. 24 in the journal Science, is that a mere four glial cells in the worm’s brain control the stress response in cells throughout its body and increase the worm’s lifespan by 75%. That was a surprise, since glial cells are often dismissed as mere support cells for the neurons that do the brain’s real work, like learning and memory. This finding follows a 2013 study in which the UC Berkeley group reported that neurons help regulate the stress response in peripheral cells, though in a different way than glial cells, and lengthen a worm’s life by about 25%. In mice, boosting neuronal regulation increases lifespan by about 10%. Together, these results paint a picture of the brain’s two-pronged approach to keeping the body’s cells healthy. When the brain senses a stressful environment — invading bacteria or viruses, for example — a subset of neurons sends electrical signals to peripheral cells to get them mobilized to respond to the stress, such as through breaking up tangles, boosting protein production and mobilizing stored fat. But because electrical signals produce only a short-lived response, the glial cells kick in to send out a long-lasting hormone, so far unidentified, that maintains a long-term, anti-stress response.
Wyss Institute, Harvard Medical School study offers hope for single genetic treatment for multiple age-related ills. New research from the Wyss Institute for Biologically Inspired Engineering at Harvard University and Harvard Medical School (HMS) suggests that it may be possible someday to tend to multiple ailments with one treatment. In the Wyss study, a single administration of an adeno-associated virus (AAV)-based gene therapy, which delivered combinations of three longevity-associated genes to mice, dramatically improved or completely reversed multiple age-related diseases, suggesting that a systems-level approach to treating such diseases could improve overall health and lifespan. The research is reported in PNAS. “The results we saw were stunning and suggest that holistically addressing aging via gene therapy could be more effective than the piecemeal approach that currently exists,” said first author Noah Davidsohn, a former research scientist at the Wyss Institute and HMS who is now chief technology officer of Rejuvenate Bio. “Everyone wants to stay as healthy as possible for as long as possible, and this study is a first step toward reducing the suffering caused by debilitating diseases.” The study was conducted in the lab of Wyss core faculty member George Church as part of Davidsohn’s postdoctoral research into the genetics of aging. Davidsohn, Church, and their co-authors homed in on three genes that had been shown to confer increased health and lifespan benefits in mice that were genetically engineered to overexpress them: FGF21, sTGFβR2, and αKlotho. They hypothesized that providing extra copies of those genes to nonengineered mice via gene therapy would similarly combat age-related diseases and bring health benefits. The team created separate gene therapy constructs for each gene using the AAV8 serotype as a delivery vehicle, and injected them into mouse models of obesity, Type 2 diabetes, heart failure, and renal failure both individually and in combination with the other genes to see whether there was a positive synergistic effect. FGF21 caused complete reversal of weight gain and Type 2 diabetes in obese, diabetic mice following a single gene therapy administration, and its combination with sTGFβR2 reduced kidney atrophy by 75 percent in mice with renal fibrosis. Heart function in mice with heart failure improved by 58 percent when they were given sTGFβR2 alone or in combination with either of the other two genes, showing that a combined therapeutic treatment of FGF21 and sTGFβR2 could successfully treat all four age-related conditions, therefore improving health and survival. Administering all three genes together resulted in slightly worse outcomes, likely from an adverse interaction between FGF21 and αKlotho, which remains to be studied.
A new study from the University of Eastern Finland shows that a moderately high intake of dietary cholesterol or consumption of up to one egg per day is not associated with an elevated risk of stroke. Furthermore, no association was found in carriers of the apolipoprotein E phenotype 4 (APOE4), which affects cholesterol metabolism and is remarkably common among the Finnish population. The findings were published in theAmerican Journal of Clinical Nutrition. Findings from earlier studies addressing the association of dietary cholesterol or egg intake with the risk of stroke have been contradictory. Some studies have found an association between high dietary cholesterol intake and an increased risk of stroke, while others have associated the consumption of eggs, which are high in cholesterol, with a reduced risk of stroke. For most people, dietary cholesterol plays a very small role in affecting their serum cholesterol levels. However, in carriers of APOE4 -- which significantly impacts cholesterol metabolism -- the effect of dietary cholesterol on serum cholesterol levels is greater. In Finland, the prevalence of APOE4, which is a hereditary variant, is exceptionally high, with approximately one third of the population presenting as carriers. Yet, research data on the association between a high intake of dietary cholesterol and the risk of stroke in this population group has not been available until now. The dietary habits of 1,950 men aged between 42 and 60 years with no baseline diagnosis of a cardiovascular disease were assessed at the onset the Kuopio Ischaemic Heart Disease Risk Factor Study, KIHD, in 1984-1989 at the University of Eastern Finland. APOE phenotype data were available for 1,015 of the men participating in the study. Of those, 32% were known carriers of APOE4. During a follow-up of 21 years, 217 men were diagnosed with stroke. The study found that neither dietary cholesterol nor egg consumption was associated with the risk of stroke -- not even in carriers of APOE4.
An enzyme-blocking molecule can extend the lifespan of Caenorhabditis elegans roundworms by as much as 45 percent, largely by modulating a cannabinoid biological pathway, according to a new study. The scientists, whose work is published on March 25 2019 in Nature Chemical Biology, also showed that the lifespan-extending cannabinoid pathway in C. elegans is related in unexpected ways to cannabinoid pathways found in humans and other mammals. "This study reveals a new life-extension pathway, but more broadly, it introduces a powerful method for applying chemical probes to lab animals such as worms to discover biology that may be relevant to humans," says study senior author Benjamin Cravatt, PhD, Professor and Gilula Chair of Chemical Biology at Scripps Research. Cravatt is known for his development of advanced "chemical proteomics" methods for studying enzymes and the biological pathways they regulate. In the new study his team deployed these methods to investigate aging in C. elegans roundworms. The tiny worms normally live for just a few weeks -- compared to two or three years for lab mice -- making them, in principle, more practical for lifespan studies. Lifespan studies using C. elegans worms typically involve the deletion or silencing of a particular gene in the embryonic stage of life to see if that extends the average lifespan of affected animals. The Cravatt team's approach, by contrast, was to use small-molecule compounds to disrupt enzyme-related pathways in adult worms, in the hope that this would uncover pathways that regulate lifespan. "The beauty of this approach is that any lifespan-extending compounds we identify can be useful tools to study whether the same mechanisms and targets also modulate aging in mammals," says study co-author Michael Petrascheck, PhD, associate professor in the Department of Molecular Medicine at Scripps Research. The team used a library of about 100 such compounds, all known to inhibit enzymes called serine hydrolases in mammals. "Metabolic processes are very important in determining the rate of aging and lifespan, and serine hydrolases are major metabolic enzymes, so we thought there was a good chance we'd find an important aging-related enzyme this way," says study first author Alice Chen, a graduate student in the Cravatt lab. After finding ways to get the compounds through the tough outer skin of the worms, Chen tested them on worms that were 1 day into adulthood, and found that some of the compounds extended average worm lifespan by at least 15 percent. One, a carbamate compound called JZL184, extended worm lifespan by 45 percent at the optimal dose. More than half the worms treated with JZL184 were still alive and apparently healthy at 30 days, a time when virtually all untreated worms were dead of old age. JZL184 was originally developed by the Cravatt lab as an inhibitor of the mammalian enzyme monoacylglycerol lipase (MAGL), whose normal job includes the breakdown of a molecule called 2-AG. The latter is an important neurotransmitter and is known as an endogenous cannabinoid ("endocannabinoid") because it activates one of the receptors hit by the main psychoactive component in cannabis. Curiously however, a corresponding MAGL enzyme does not exist in C. elegans worms, so JZL184's target in these animals was a mystery. Chen soon found, though, that one of the main target enzymes for JZL184 in worms was fatty acid amide hydrolase 4 (FAAH-4). Although FAAH-4 and MAGL are not related in terms of their amino-acid sequences or 3-D folds, further experiments revealed, surprisingly, that FAAH-4 in worms does what MAGL does in humans and other mammals: it breaks down 2-AG. 2-AG has been linked to aging in mammals; one recent study found evidence that its levels fall in the brains of aging mice, likely due to greater MAGL activity. The results suggest, then, that studying the FAAH-4/2-AG pathway in worms could one day yield lifespan-extending strategies for humans. "It seems at least plausible at this point that both worms and mammals have a cannabinoid-related signaling pathway that affects longevity and possibly aging-related disorders," Cravatt says. The study demonstrates more generally how libraries of small-molecule compounds and associated proteomics techniques can be used to reveal biological pathways that evolutionarily distant lab animals such as worms have in common with humans. "In principle, with this approach one can quickly find a compound that has a desired biological effect and also find the target through which it works, all in a live and relatively complex model organism," Cravatt says.
|
Aging, or senescent cells, which stop dividing but don’t die, can accumulate in the body over the years and fuel chronic inflammation that contributes to conditions such as cancer and degenerative disorders. In mice, eliminating senescent cells from aging tissues can restore tissue balance and lead to an increased healthy lifespan. Now a team led by investigators at Massachusetts General Hospital (MGH) has found that the immune response to a virus that is ubiquitously present in human tissues can detect and eliminate senescent cells in the skin. For the study, which is published in Cell, the scientists analyzed young and old human skin samples to learn more about the clearance of senescent cells in human tissue. The researchers found more senescent cells in the old skin compared with young skin samples. However, in the samples from old individuals, the number of senescent cells did not increase as individuals got progressively older, suggesting that some type of mechanism kicks in to keep them in check. Experiments suggested that once a person becomes elderly, certain immune cells called killer CD4+ T cells are responsible for keeping senescent cells from increasing. Indeed, higher numbers of killer CD4+ T cells in tissue samples were associated with reduced numbers of senescent cells in old skin. When they assessed how killer CD4+ T cells keep senescent cells in check, the researchers found that aging skin cells express a protein, or antigen, produced by human cytomegalovirus, a pervasive herpesvirus that establishes lifelong latent infection in most humans without any symptoms. By expressing this protein, senescent cells become targets for attack by killer CD4+ T cells. “Our study has revealed that immune responses to human cytomegalovirus contribute to maintaining the balance of aging organs,” says senior author Shawn Demehri, MD, PhD, director of the High Risk Skin Cancer Clinic at MGH and an associate professor of Dermatology at Harvard Medical School. “Most of us are infected with human cytomegalovirus, and our immune system has evolved to eliminate cells, including senescent cells, that upregulate the expression of cytomegalovirus antigens.” These findings, which highlight a beneficial function of viruses living in our body, could have a variety of clinical applications. “Our research enables a new therapeutic approach to eliminate aging cells by boosting the anti-viral immune response,” says Demehri. “We are interested in utilizing the immune response to cytomegalovirus as a therapy to eliminate senescent cells in diseases like cancer, fibrosis and degenerative diseases.” Demehri notes that the work may also lead to advances in cosmetic dermatology, for example in the development of new treatments to make skin look younger.
Via Juan Lama
Recent lab studies have shown that aging is a reversible process, an advancement that has prompted scientists to seek ways to stop the functional decline of cells and tissues, as well as restore their regenerative capacity. This includes researchers at the University at Buffalo, where chemical engineer Stelios Andreadis showed that the embryonic gene NANOG could reprogram senescent (aged) adult stem cells and skeletal muscle cells, thereby reversing the hallmarks of aging. How exactly NANOG works, though, has been a mystery. Now, two new studies from Andreadis’ lab are helping to answer this question. One in Cell Reports explores the role NANOG plays in restoring mitochondrial function in aging stem cells. The other, published Feb. 16 in Nature Communications, sheds light on how it reverses aging in skeletal muscle. The works builds upon the scientific community’s understanding of NANOG, which is named for the mythical land of youth in Irish folklore, and could help lead to the development of medicines that mimic the gene. “With these studies, we discovered that NANOG reverses cellular senescence by restoring metabolic pathways that are active in younger cells. This brings us closer to developing improved treatments that will help alleviate suffering worldwide for people struggling with age-related illnesses,” said Andreadis, PhD, at SUNY.
An international team of 114 scientists, led by Penn State and Northeastern Illinois University, reports the most comprehensive study of aging and longevity to date comprising data collected in the wild from 107 populations of 77 species of reptiles and amphibians worldwide. At 190 years old, Jonathan the Seychelles giant tortoise recently made news for being the "oldest living land animal in the world." Although, anecdotal evidence like this exists that some species of turtles and other ectotherms -- or 'cold-blooded' animals -- live a long time, evidence is spotty and mostly focused on animals living in zoos or a few individuals living in the wild. Now, an international team of 114 scientists, led by Penn State and Northeastern Illinois University, reports the most comprehensive study of aging and longevity to date comprising data collected in the wild from 107 populations of 77 species of reptiles and amphibians worldwide. Among their many findings, which they published in the journal Science, the researchers documented for the first time that turtles, crocodilians and salamanders have particularly low aging rates and extended lifespans for their sizes. The team also found that protective phenotypes, such as the hard shells of most turtle species, contribute to slower aging, and in some cases even 'negligible aging' -- or lack of biological aging. "Anecdotal evidence exists that some reptiles and amphibians age slowly and have long lifespans, but until now no one has actually studied this on a large scale across numerous species in the wild," said David Miller, senior author and associate professor of wildlife population ecology, Penn State. "If we can understand what allows some animals to age more slowly, we can better understand aging in humans, and we can also inform conservation strategies for reptiles and amphibians, many of which are threatened or endangered." In their study, the researchers applied comparative phylogenetic methods -- which enable investigation of organisms' evolution -- to mark-recapture data -- in which animals are captured, tagged, released back into the wild and observed. Their goal was to analyze variation in ectotherm aging and longevity in the wild compared to endotherms (warm-blooded animals) and explore previous hypotheses related to aging -- including mode of body temperature regulation and presence or absence of protective physical traits. Miller explained that the 'thermoregulatory mode hypothesis' suggests that ectotherms -- because they require external temperatures to regulate their body temperatures and, therefore, often have lower metabolisms -- age more slowly than endotherms, which internally generate their own heat and have higher metabolisms. "People tend to think, for example, that mice age quickly because they have high metabolisms, whereas turtles age slowly because they have low metabolisms," said Miller. The team's findings, however, reveal that ectotherms' aging rates and lifespans range both well above and below the known aging rates for similar-sized endotherms, suggesting that the way an animal regulates its temperature -- cold-blooded versus warm-blooded -- is not necessarily indicative of its aging rate or lifespan. "We didn't find support for the idea that a lower metabolic rate means ectotherms are aging slower," said Miller. "That relationship was only true for turtles, which suggests that turtles are unique among ectotherms." The protective phenotypes hypothesis suggests that animals with physical or chemical traits that confer protection -- such as armor, spines, shells or venom -- have slower aging and greater longevity. The team documented that these protective traits do, indeed, enable animals to age more slowly and, in the case of physical protection, live much longer for their size than those without protective phenotypes. "It could be that their altered morphology with hard shells provides protection and has contributed to the evolution of their life histories, including negligible aging -- or lack of demographic aging -- and exceptional longevity," said Anne Bronikowski, co-senior author and professor of integrative biology, Michigan State. Beth Reinke, first author and assistant professor of biology, Northeastern Illinois University, further explained, "These various protective mechanisms can reduce animals' mortality rates because they're not getting eaten by other animals. Thus, they're more likely to live longer, and that exerts pressure to age more slowly. We found the biggest support for the protective phenotype hypothesis in turtles. Again, this demonstrates that turtles, as a group, are unique." Interestingly, the team observed negligible aging in at least one species in each of the ectotherm groups, including in frogs and toads, crocodilians and turtles. "It sounds dramatic to say that they don't age at all, but basically their likelihood of dying does not change with age once they're past reproduction," said Reinke. Miller added, "Negligible aging means that if an animal's chance of dying in a year is 1% at age 10, if it is alive at 100 years, it's chance of dying is still 1% (1). By contrast, in adult females in the U.S., the risk of dying in a year is about 1 in 2,500 at age 10 and 1 in 24 at age 80. When a species exhibits negligible senescence (deterioration), aging just doesn't happen."
Research from the Babraham Institute has developed a method to ‘time jump’ human skin cells by 30 years, turning back the aging clock for cells without losing their specialized function. Work by researchers in the Institute’s Epigenetics research program has been able to partly restore the function of older cells, as well as rejuvenating the molecular measures of biological age. The research is published in the journal eLife and whilst at an early stage of exploration, it could revolutionize regenerative medicine. How does it work? As we age, our cells’ ability to function declines and the genome accumulates marks of aging. Regenerative biology aims to repair or replace cells including old ones. One of the most important tools in regenerative biology is our ability to create ‘induced’ stem cells. The process is a result of several steps, each erasing some of the marks that make cells specialized. In theory, these stem cells have the potential to become any cell type, but scientists aren’t yet able to reliably recreate the conditions to re-differentiate stem cells into all cell types. The new method, based on the Nobel Prize winning technique scientists use to generate stem cells, overcomes the problem of entirely erasing cell identity by halting reprogramming part of the way through the whole process. This allowed researchers to find the precise balance between reprogramming cells, making them biologically younger, while still being able to regain their specialized cell function. In 2007, Shinya Yamanaka was the first scientist to turn normal cells, which have a specific function, into stem cells which have the special ability to develop into any cell type. The full process of stem cell reprogramming takes around 50 days using four key molecules called the Yamanaka factors. The new method, called 'maturation phase transient reprogramming’, exposes cells to Yamanaka factors for just 13 days. At this point, age-related changes are removed and the cells have temporarily lost their identity. The partly reprogrammed cells were given time to grow under normal conditions, to observe whether their specific skin cell function returned. Genome analysis showed that cells had regained markers characteristic of skin cells (fibroblasts), and this was confirmed by observing collagen production in the reprogrammed cells.
Contrary to what science once suggested, older people with a declining sense of smell do not have comprehensively dampened olfactory ability for odors in general -- it simply depends upon the type of odor. Researchers at the University of Copenhagen reached this conclusion after examining a large group of older Danes' and their intensity perception of common food odors. That older people aren't as good at smelling as they once were, is something that many can relate to. And, it has also been scientifically demonstrated. One's sense of smell gradually begins to decline from about the age of 55. Until now, it was believed that one's sense of smell broadly declined with increasing age. However, a study from the University of Copenhagen reports that certain food odors are significantly more affected than others. The Department of Food Science's Eva Honnens de Lichtenberg Broge and her fellow researchers have tested the ability of older Danes to perceive everyday food odors. The researchers measured how intensely older adults perceived different food odors, as well as how much they liked the odors. "Our study shows that the declining sense of smell among older adults is more complex than once believed. While their ability to smell fried meat, onions and mushrooms is markedly weaker, they smell orange, raspberry and vanilla just as well as younger adults. Thus, a declining sense of smell in older adults seems rather odor specific. What is really interesting is that how much you like an odor is not necessarily dependent on the intensity perception" says Eva Honnens de Lichtenberg Broge. For example, liking of seemed to be largely unaffected for fried meat, onions and mushrooms, despite the largest decline in intensity perception was seen for these specific odors. Also the ability to smell coffee declined, among other things, though they didn't like the aroma of coffee to the same degree as younger adults. The test subjects included 251 Danes between the ages of 60 and 98 and a control group consisting of 92 people between the ages of 20 and 39.
Even though people tend to remember fewer details about past events as time goes by, the details they do remember are retained with remarkable fidelity, according to a new study. This finding holds true regardless of the age of the person or the amount of time that elapsed since the event took place.
Scientists studying the complex relationship between aging and memory have found that in a controlled experiment, people can remember the details about past events with a surprising 94% accuracy, even accounting for age. These results, published in the journal Psychological Science, suggest that the stories we tell about past events are accurate, although details tend to fade with time.
“These results are surprising to many, given the general pessimism about memory accuracy among scientists and the prevalent idea that memory for one-time events is not to be trusted,” said Nicholas Diamond, the study’s lead researcher, a former graduate student at Baycrest’s Rotman Research Institute (RRI), and currently a postdoctoral researcher at the University of Pennsylvania.
“These results will be helpful for understanding memory in healthy aging.”Brian Levine, Baycrest’s Rotman Research Institute
About 400 academics, including memory scientists, surveyed as part of this study estimated memory accuracy to be around 40% at best, expecting this score to be even lower for older participants or when greater amounts of time had elapsed since the events.
“This study shows us that memory accuracy is actually quite good under normal circumstances, and it remains stable as we age,” said Brian Levine, a senior scientist at RRI and a professor of psychology and neurology at the University of Toronto and co-author on the study. “These results will be helpful for understanding memory in healthy aging.”
Scientists have estimated that the age of an individual does not indicate how likely they are to be infected by SARS-CoV-2. However, development of symptoms, progression of the disease, and mortality are age-dependent. There have been a large number of deaths due to the ongoing COVID-19 pandemic, and it has been shown that elderly individuals disproportionately develop severe symptoms and show higher mortality. A team of scientists, including Associate Professor Ryosuke Omori from the Research Center for Zoonoses Control at Hokkaido University, have modeled available data from Japan, Spain and Italy to show that susceptibility to COVID-19 is independent of age, while occurrence of symptomatic COVID-19, severity and mortality is likely dependent on age. Their results were published in the journal Scientific Reports on October 6, 2020. Causes of mortality in elderly individuals may be due to two factors: how likely they are to be infected due to their advanced age (age-dependent susceptibility), which is reflected in the number of cases; and, how likely they will be affected by a severe form of the disease due to their advanced age (age-dependent severity), which is reflected in the mortality rate. These factors are not fully understood for COVID-19. The scientists chose to analyse data from Italy, Spain and Japan to determine if any relationship between age, susceptibility and severity. These three countries were chosen as they have well recorded, publicly available data. As of May 2020, the mortality rate (number of deaths per 100,000) was 382.3 for Italy, 507.2 for Spain and 13.2 for Japan. However, despite the wide disparity in mortality rates, the age distribution of mortality (the proportional number of deaths per age group) was similar for these countries.
The WSU team’s findings suggest that Washingtonians who live in highly walkable, mixed‑age communities may be more likely to live to their 100th birthday. When it comes to living to the ripe old age of 100, good genes help but don’t tell the full story. Where you live has a significant impact on the likelihood that you will reach centenarian age, suggests a new study conducted by scientists at Washington State University’s Elson S. Floyd College of Medicine. Published in the International Journal of Environmental Research and Public Health and based on Washington State mortality data, the research team’s findings suggest that Washingtonians who live in highly walkable, mixed-age communities may be more likely to live to their 100th birthday. They also found socioeconomic status to be correlated, and an additional analysis showed that geographic clusters where the probability of reaching centenarian age is high are located in urban areas and smaller towns with higher socioeconomic status, including the Seattle area and the region around Pullman, Washington.
Old human cells return to a more youthful and vigorous state after being induced to briefly express a panel of proteins involved in embryonic development, according to a new study by researchers at the Stanford University School of Medicine. The researchers also found that elderly mice regained youthful strength after their existing muscle stem cells were subjected to the rejuvenating protein treatment and transplanted back into their bodies. The proteins, known as Yamanaka factors, are commonly used to transform an adult cell into what are known as induced pluripotent stem cells, or iPS cells. Induced pluripotent stem cells can become nearly any type of cell in the body, regardless of the cell from which they originated. They've become important in regenerative medicine and drug discovery. The study found that inducing old human cells in a lab dish to briefly express these proteins rewinds many of the molecular hallmarks of aging and renders the treated cells nearly indistinguishable from their younger counterparts. "When iPS cells are made from adult cells, they become both youthful and pluripotent," said Vittorio Sebastiano, PhD, assistant professor of obstetrics and gynecology and the Woods Family Faculty Scholar in Pediatric Translational Medicine. "We've wondered for some time if it might be possible to simply rewind the aging clock without inducing pluripotency. Now we've found that, by tightly controlling the duration of the exposure to these protein factors, we can promote rejuvenation in multiple human cell types." Sebastiano is the senior author of the study, which will be published online March 24 in Nature Communications. Former graduate student Tapash Sarkar, PhD, is the lead author of the article. "We are very excited about these findings," said study co-author Thomas Rando, MD, PhD, professor of neurology and neurological sciences and the director of Stanford's Glenn Center for the Biology of Aging. "My colleagues and I have been pursuing the rejuvenation of tissues since our studies in the early 2000s revealed that systemic factors can make old tissues younger. In 2012, Howard Chang and I proposed the concept of using reprogramming factors to rejuvenate cells and tissues, and it is gratifying to see evidence of success with this approach." Chang, MD, PhD, is a professor of dermatology and of genetics at Stanford. Exposure to proteins Researchers in Sebastiano's laboratory make iPS cells from adult cells, such as those that compose skin, by repeatedly exposing them over a period of about two weeks to a panel of proteins important to early embryonic development. They do so by introducing daily, short-lived RNA messages into the adult cells. The RNA messages encode the instructions for making the Yamanaka proteins. Over time, these proteins rewind the cells' fate -- pushing them backward along the developmental timeline until they resemble the young, embryonic-like pluripotent cells from which they originated. During this process the cells not only shed any memories of their previous identities, but they revert to a younger state. They accomplish this transformation by wiping their DNA clean of the molecular tags that not only differentiate, say, a skin cell from a heart muscle cell, but of other tags that accumulate as a cell ages. Recently researchers have begun to wonder whether exposing the adult cells to Yamanaka proteins for days rather than weeks could trigger this youthful reversion without inducing full-on pluripotency. In fact, researchers at the Salk Institute for Biological Studies found in 2016 that briefly expressing the four Yamanaka factors in mice with a form of premature aging extended the animals' life span by about 20%. But it wasn't clear whether this approach would work in humans. Sarkar and Sebastiano wondered whether old human cells would respond in a similar fashion, and whether the response would be limited to just a few cell types or generalizable for many tissues. They devised a way to use genetic material called messenger RNA to temporarily express six reprogramming factors -- the four Yamanaka factors plus two additional proteins -- in human skin and blood vessel cells. Messenger RNA rapidly degrades in cells, allowing the researchers to tightly control the duration of the signal. The researchers then compared the gene-expression patterns of treated cells and control cells, both obtained from elderly adults, with those of untreated cells from younger people. They found that cells from elderly people exhibited signs of aging reversal after just four days of exposure to the reprogramming factors. Whereas untreated elderly cells expressed higher levels of genes associated with known aging pathways, treated elderly cells more closely resembled younger cells in their patterns of gene expression. When the researchers studied the patterns of aging-associated chemical tags called methyl groups, which serve as an indicator of a cell's chronological age, they found that the treated cells appeared to be about 1½ to 3½ years younger on average than untreated cells from elderly people, with peaks of 3½ years (in skin cells) and 7½ years (in cells that line blood vessels).
Scientists at the MDI Biological Laboratory, in collaboration with scientists from the Buck Institute for Research on Aging in Novato, Calif., and Nanjing University in China, have identified synergistic cellular pathways for longevity that amplify lifespan fivefold in C. elegans, a nematode worm used as a model in aging research. The increase in lifespan would be the equivalent of a human living for 400 or 500 years, according to one of the scientists. The research draws on the discovery of two major pathways governing aging in C. elegans, which is a popular model in aging research because it shares many of its genes with humans and because its short lifespan of only three to four weeks allows scientists to quickly assess the effects of genetic and environmental interventions to extend healthy lifespan. Because these pathways are “conserved,” meaning that they have been passed down to humans through evolution, they have been the subject of intensive research. A number of drugs that extend healthy lifespan by altering these pathways are now under development. The discovery of the synergistic effect opens the door to even more effective anti-aging therapies. The new research uses a double mutant in which the insulin signaling (IIS) and TOR pathways have been genetically altered. Because alteration of the IIS pathways yields a 100 percent increase in lifespan and alteration of the TOR pathway yields a 30 percent increase, the double mutant would be expected to live 130 percent longer. But instead, its lifespan was amplified by 500 percent. “Despite the discovery in C. elegans of cellular pathways that govern aging, it hasn’t been clear how these pathways interact,” said Hermann Haller, M.D., president of the MDI Biological Laboratory. “By helping to characterize these interactions, our scientists are paving the way for much-needed therapies to increase healthy lifespan for a rapidly aging population.”
The elucidation of the cellular mechanisms controlling the synergistic response is the subject of a recent paper in the online journal Cell Reports entitled “Translational Regulation of Non-autonomous Mitochondrial Stress Response Promotes Longevity.” The authors include Jarod A. Rollins, Ph.D., and Aric N. Rogers, Ph.D., of the MDI Biological Laboratory.
“The synergistic extension is really wild,” said Rollins, who is the lead author with Jianfeng Lan, Ph.D., of Nanjing University. “The effect isn’t one plus one equals two, it’s one plus one equals five. Our findings demonstrate that nothing in nature exists in a vacuum; in order to develop the most effective anti-aging treatments we have to look at longevity networks rather than individual pathways.”
Scientists once thought that neurons, or possibly heart cells, were the oldest cells in the body. Now, Salk Institute researchers have discovered that the mouse brain, liver and pancreas contain populations of cells and proteins with extremely long lifespans -- some as old as neurons. The findings, demonstrating "age mosaicism," were published in Cell Metabolismon June 6, 2019. The team's methods could be applied to nearly any tissue in the body to provide valuable information about lifelong function of non-dividing cells and how cells lose control over the quality and integrity of proteins and important cell structures during aging. "We were quite surprised to find cellular structures that are essentially as old as the organism they reside in," says Salk Vice President, Chief Science Officer Martin Hetzer, senior author and professor. "This suggests even greater cellular complexity than we previously imagined and has intriguing implications for how we think about the aging of organs, such as the brain, heart and pancreas." Most neurons in the brain do not divide during adulthood and thus experience a long lifespan and age-related decline. Yet, largely due to technical limitations, the lifespan of cells outside of the brain was difficult to determine. "Biologists have asked -- how old are cells in an organism? There is this general idea that neurons are old, while other cells in the body are relatively young and regenerate throughout the organism's lifetime," says Rafael Arrojo e Drigo, first author and Salk staff scientist. "We set out to see if it was possible that certain organs also have cells that were as long-lived as neurons in the brain." Since the researchers knew that most neurons are not replaced during the lifespan, they used them as an "age baseline" to compare other non-dividing cells. The team combined electron isotope labeling with a hybrid imaging method (MIMS-EM) to visualize and quantify cell and protein age and turnover in the brain, pancreas and liver in young and old rodent models.
Explorers have dreamt for centuries of a Fountain of Youth, with healing waters that rejuvenate the old and extend life indefinitely. SIRT6 is often called the "longevity gene" because of its important role in organizing proteins and recruiting enzymes that repair broken DNA; additionally, mice without the gene age prematurely, while mice with extra copies live longer. Researchers have long hypothesized that if more efficient DNA repair is required for a longer lifespan, organisms with longer lifespans may have evolved more efficient DNA repair regulators. Is SIRT6 activity therefore enhanced in longer-lived species? To test this theory, the researchers analyzed DNA repair in 18 rodent species with lifespans ranging from 3 years (mice) to 32 years (naked mole rats and beavers). Scientists found that the rodents with longer lifespans also experience more efficient DNA repair because the products of their SIRT6 genes—the SIRT6 proteins—are more potent. That is, SIRT6 is not the same in every species. Instead, the gene has co-evolved with longevity, becoming more efficient so that species with a stronger SIRT6 live longer. "The SIRT6 protein seems to be the dominant determinant of lifespan," Bohmann, one of the scientists, says. "We show that at the cell level, the DNA repair works better, and at the organism level, there is an extended lifespan." The researchers then analyzed the molecular differences between the weaker SIRT6 protein found in mice versus the stronger SIRT6 found in beavers. They identified five amino acids responsible for making the stronger SIRT6 protein "more active in repairing DNA and better at enzyme functions," one member of the study says. When the researchers inserted beaver and mouse SIRT6 into human cells, the beaver SIRT6 better reduced stress-induced DNA damage compared to when researchers inserted the mouse SIRT6. The beaver SIRT6 also better increased the lifespan of fruit flies versus fruit flies with mouse SIRT6. Species with even more robust SIRT6? Although it appears that human SIRT6 is already optimized to function, "we have other species that are even longer lived than humans," another study member says. Next steps in the research involve analyzing whether species that have longer lifespans than humans—like the bowhead whale, which can live more than 200 years—have evolved even more robust SIRT6 genes.
|



 Your new post is loading...
Your new post is loading...



















Buy Pethidine online
Price: $190.0 – $480.0Buy Prozac Online
Price: $100.0 – $250.0buy PURE WHEY PROTEIN online
Buy quaaludes online
Buy quality Ativan online
Buy quality methadone online
Price: $150.0 – $900.0Buy quality vyvanse online
Price: $200.0 – $450.0Buy sibelium online
Price: $150.0 – $250.0buy Sodium valproate
buy suboxone strips online
Price: $160.0 – $500.0Buy Subutex Online
Price: $180.0 – $450.0Buy to quality roxicodone Online
Price: $240.0 – $600.0Buy TOP QUALITY Suboxone Online
Price: $150.0 – $500.0Buy Ultram Online
Price: $100.0 – $400.0Buy vicodin online
Price: $150.0 – $500.0Buy vicoprofen online