Genetic changes to avian influenza viruses have led to spread among many wild species, creating an uncontrollable global outbreak. Researchers studying the evolution of the bird flu virus over the past 18 years have shown how the strain currently circulating worldwide, an extremely deadly form of the H5N1 subtype, has become increasingly infectious to wild birds. The strain emerged in Europe in 2020, and has spread to an unprecedented number of countries. The study, published in Nature on the 18th October 2023 looked at changes to the virus’s genome over time and used data on reported outbreaks to track how it spread. In 2020, the rate of spread among wild birds was three times faster than that in farmed poultry, because of mutations that allowed the virus to adapt to diverse species. “What was once very clearly a poultry pathogen has now become an animal-health issue much more broadly,” says Andy Ramey, a wildlife geneticist at the US Geological Survey Alaska Science Center in Anchorage. “That has implications for wildlife and domestic poultry as well as us humans that rely upon these resources.”
Persistent outbreaks
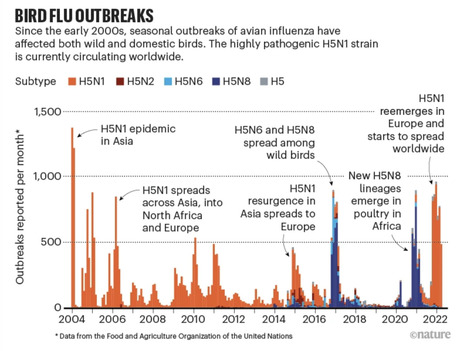
H5N1, classified as a highly pathogenic avian influenza (HPAI) virus because of its high death toll in poultry, was first detected in birds in China in 1996. Outbreaks are usually seasonal, synchronizing with bird migration in Northern Hemisphere autumn. But since November 2021, they have become persistent. In 2022, the virus killed millions of birds across five continents and seeded outbreaks among farmed mink and various marine mammals. To study changes in the virus’s behaviour, the authors examined data reported to the Food and Agricultural Organization of the United Nations and the World Organisation for Animal Health between 2005 and 2022, and analysed more than 10,000 viral genomes. Their work reveals that in mid-2020, a new H5N1 strain evolved from an earlier variety, called H5N8, which first emerged in poultry in Egypt between 2016 and 2017 and caused global flare-ups throughout 2020 and 2021 (see ‘Bird flu outbreaks’). The new H5N1 virus mutated through interactions with non-deadly varieties of bird flu, called low-pathogenic avian influenza (LPAI) viruses, that had been circulating among wild birds in Europe since 2019. It developed two subtypes in 2021 and 2022. One spread across the northern coastal regions of central Europe and was eventually carried to North America by birds migrating across the Atlantic Ocean. The other was carried around the Mediterranean Sea and into Africa. Many bird flu outbreaks begin in poultry, but spillover into wild birds has spread the disease into larger areas, creating a global challenge that is difficult to manage, the study found. “Once it’s adapted to wild birds, we have no mechanism to control the virus. And I think that’s the biggest impact that has changed now,” says co-author Vijaykrishna Dhanasekaran, an evolutionary biologist and virologist at the University of Hong Kong. Louise Moncla, an evolutionary virologist at the University of Pennsylvania in Philadelphia, agrees. “Regardless of how much outbreak response you do in poultry, if it’s coming in from wild birds repeatedly, this is going to be really hard to manage.” “This is really something that most of the world at this point has skin in the game,” adds Ramey.
Mixing viruses
LPAI viruses often circulate freely in poultry and wild birds. Previous infection with these non-deadly strains is thought to encourage population immunity in wild birds. “You can think of it as an imperfect vaccine, that doesn’t stop infection, but it helps mitigate the effects of disease,” says Ramey. But “there’s probably two sides of the coin here”, he adds. HPAI viruses can mutate through interactions with LPAI ones. In both, the genome is split into eight segments that can be mixed and matched. “When two viruses co-infect the same cell, they could swap their genes when the virus is getting packaged,” says Dhanasekaran. Because of this, LPAI viruses — especially a strain called H9N2 — play a major part in the evolution of H5N1, he adds. But they are not well monitored. “Eradication or elimination strategies that target these low pathogenic viruses would be a huge step forward in terms of controlling avian influenza itself,” says Dhanasekaran.
Research Cited published in Nature (Oct. 18, 2023):
https://doi.org/10.1038/s41586-023-06631-2
Via Juan Lama



 Your new post is loading...
Your new post is loading...







Buy Klonopin without prescription
buy liquid morphine online
Buy Morphine online uk
Buy Norco Online Without Prescription
Buy Oramorph without prescription
Buy oxycontin online uk
Buy Quaaludes Online
buy sildenafil 50 mg tablet
Buy yellow Xanax online
Dilaudid for pain
Order Acxion phentermine 30mg
Order codeine without prescription