Your new post is loading...
 Your new post is loading...

|
Scooped by
iBB
July 28, 2015 10:29 AM
|
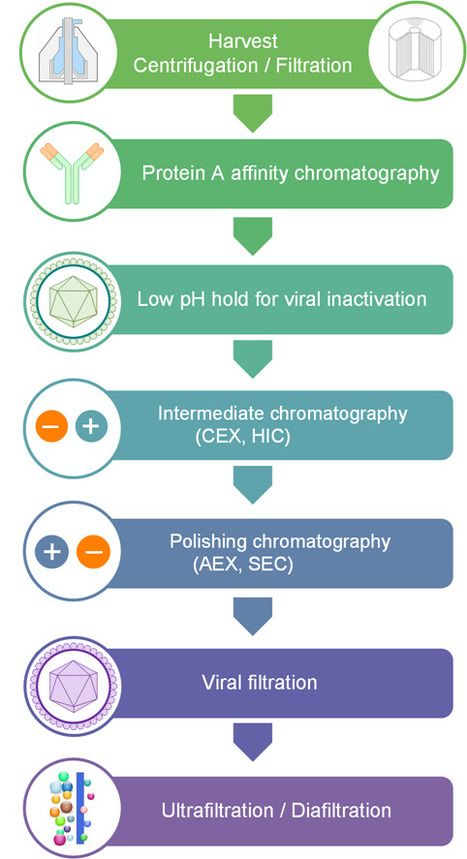
The commercial potential of monoclonal antibodies (mAbs) has been continuously increasing during the last years alongside with the number of approved mAb-based drugs and clinical trials. Despite their effectiveness and safety, the general access to this class of biopharmaceuticals is barred by high selling prices. Downstream processing is now considered the bottleneck in the manufacturing of mAbs. Therefore, the design of novel and economic operations and their implementation in the current technology platforms constitutes a pressing need. A recent review published by researchers from the Bioseparation Engineering Laboratory at BERG-iBB, provides an insight into the current state-of-the-art in mAbs purification, focusing on multimodal chromatography as one of the viable options to upgrade the established purification train. Multimodal chromatography has been receiving considerable attention over the last years, with about 152 publications published in ISI-index journals in the last decade (2004-2013). The number of publications per year has been steadily increasing, with a paper-boom starting in 2008.

|
Scooped by
iBB
July 7, 2015 9:54 AM
|
The therapeutic use of human mesenchymal stem/stromal cells (MSC) for a wide range of diseases is at the front line of stem cell-based cellular therapies. Through a bioprocess engineering approach, researchers at BERG-iBB led by Cláudia Lobato Silva and Joaquim Cabral, were able to maximize the production of MSC from different human sources – bone marrow and adipose tissue - in a microcarrier-based stirred culture system. The advances, reported in a recent paper on Biotechnology Journal, clearly improve the scalability and cost-effectiveness of MSC manufacturing, while facilitating the translation to a Good Manufacturing Practices (GMP)-compliant bioprocess. Click on title to learn more.

|
Scooped by
iBB
June 12, 2015 5:23 AM
|
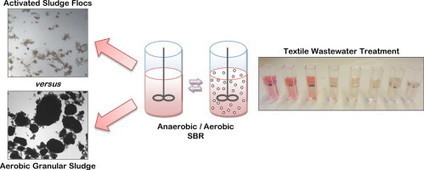
Aerobic granular sludge (AGS) is a compact and energy-saving biotechnology pointed as the next generation of wastewater treatment. In a recent publication, BERG-iBB and BSRG-iBB researchers led by Nídia Lourenço compare the performance of AGS with conventional activated sludge in the treatment of textile industry wastewater. High efficiencies were attained in both systems, with AGS providing higher yields and excellent biomass settling properties. Yeast-based toxicity assays also highlighted the better wastewater detoxification performance of AGS, supporting its potential application for industrial wastewater treatment as an effective alternative to the conventional technology based on floc-forming bacteria.

|
Scooped by
iBB
June 7, 2015 2:25 PM
|
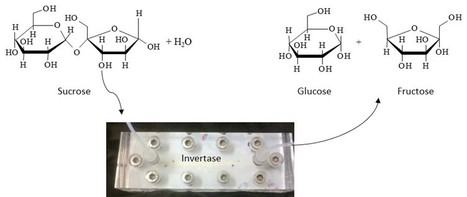
Microreactors have been gaining increased relevance as preferred process intensification tools. Microreactors present several advantages over conventional batch reactors, namely mass and heat transfer, high surface to volume ratios, minute consumption of chemicals, ease of automation and better process control. Within the scope of this exciting area of research, Pedro Fernandes and Filipe Carvalho from BERG-iBB recently designed, assembled and run a low-cost continuous flow enzyme microreactor, allowing for detailed characterization of biocatalytic systems with immobilized enzymes in particulate form. Moreover, the microreactor enabled continuous enzymatic sucrose hydrolysis for 1 month, with sustained substrate conversion of roughly 100%.

|
Scooped by
iBB
May 30, 2015 5:05 AM
|
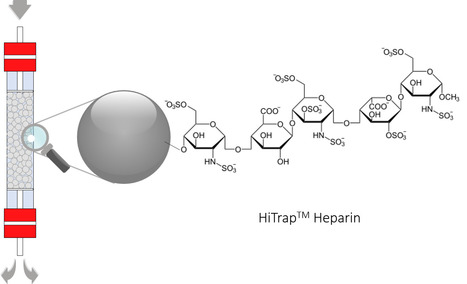
Heparin is a naturally occurring sulfonated glycosaminoglycan, characterized by a high density and distribution of negative charges that is widely used as an injectable anticoagulant. In a recent pubication, BERG-iBB researchers led by Ana Azevedo explore the strong cation exchanger potential of an heparin resin, namely its ability to capture a basic mAb from CHO cell culture supernatants. The performance of the proposed capture step was estimated in terms of yield, protein purity and purification factor, and compared to the traditional protein A affinity chromatography.
Click on title to learn more.

|
Scooped by
iBB
October 22, 2014 1:56 PM
|
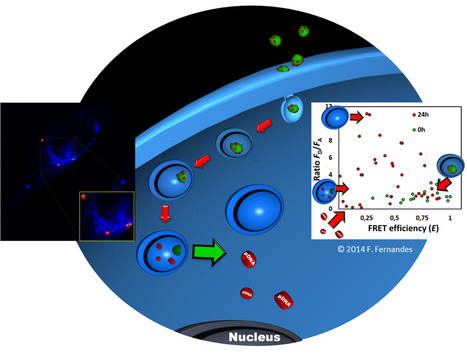
The success of gene delivery through liposomes depends on the effectiveness of endosomal escape and nucleic acid release from the chosen vectors. Performing a simultaneous quantitative monitoring of the two processes is not trivial, and most studies seeking to characterize these processes fail to do so. In a recent publication in Human Gene Therapy Methods, BERG-iBB researchers working in collaboration with Centro de Química Física Molecular of IST, describe a tool that could offer simultaneous quantitative information on both the intracellular dissociation of lipoplexes, and on the DNA escape from endocytic compartments. This ability is expected to be of high value in the rationalization and conception of new lipid nanoparticle vectors for gene delivery for therapeutic purposes. Click on title to learn more.

|
Scooped by
iBB
October 7, 2014 1:25 PM
|
Money is usually considered a source of infections. In a study comparing the bacterial numbers and diversity on coins collected in Casablanca and Lisbon, Carla C.C.R. de Carvalho (BERG-iBB, IST) and Maria-José Caramujo (FCUL) showed that coins transported in pockets presented higher number of bacterial isolates than coins transported in wallets, regardless of the gender of the person transporting them. The work, published in FEMS Microbiology Ecology, suggests that temperature and moisture might be key parameters for bacterial survival on metallic coins. The percentage of Staphylococcus strains was, curiously, 44% of the total isolates on both Moroccan Dirham and Euro coins. Click on title to learn more.

|
Scooped by
iBB
August 29, 2014 11:58 AM
|
Bacterial adaptation studies are usually performed with growing populations of cells where de novo synthesis is possible and thus the impact of the stress agent is determined on the daughter cells. In a study led by Carla C.C.R. de Carvalho (BERG-IBB, IST, Portugal) and Hermann Heipieper (UFZ, Leipizig, Germany), published in Applied Microbiology and Biotechnology, the responses of Rhodococcus erythropolis cells within the first 30 min after exposure to osmotic stress caused by NaCl were studied. Surprisingly, the exposed cells produced polyunsaturated fatty acids (PUFAs). The fact that PUFAs were detected already after 6 min of exposure to salt and their high abundance after 35 min exposure had not been described in bacteria before. Click on title to learn more.

|
Scooped by
iBB
August 2, 2014 10:21 AM
|
Researchers from BERG and Biotrend have reported for the first time the ability of Burkholderia sacchari to produce poly(3-hydroxybutyrate co-4-hydroxybutyrate) - P(3HB-co-4HB) - from xylose-rich wheat straw hydrolysates (WSH) using gamma butyrolactone (GBL) as precursor. In a joint publication in the International Journal of Biological Macromolecules, members from the two teams describe a fed-batch process that achieves high copolymer productivities (0.5 g/L.h) and 4HB incorporations (5.0 molar%) using WSH and GBL.. Due to their properties, polymers like P(3HB-co-4HB) may replace petrochemically produced bulk plastics like polyethylene and polypropylene. The fact that they are completely degradable to carbon dioxide and water through natural microbiological mineralization constitute additional advantages of PHB derivatives.

|
Scooped by
iBB
July 29, 2014 11:58 AM
|
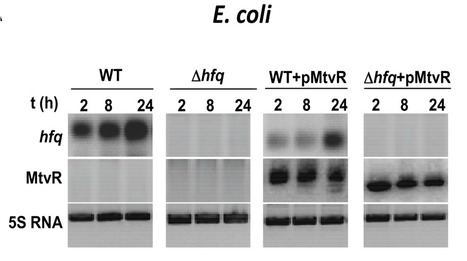
MtvR is a 136-nucleotide long sRNA previously identified in the human pathogen Burkholderia cenocepacia J2315 and with homologues restricted to bacteria of the Burkholderia cepacia complex. In a paper published in PlosOne, a team led by Jorge Leitão from BSRG show that MtvR negatively regulates the hfq mRNA levels in both bacterial species. In the case of E. coli, this negative regulation is shown to involve binding of MtvR to the 5′-UTR region of the hfqEc mRNA. Results presented also show that expression of MtvR in E. coli and P. aeruginosa originates multiple phenotypes, including reduced resistance to selected stresses, biofilm formation ability, and increased susceptibility to various antibiotics. Click on title to learn more.

|
Scooped by
iBB
July 23, 2014 12:55 PM
|
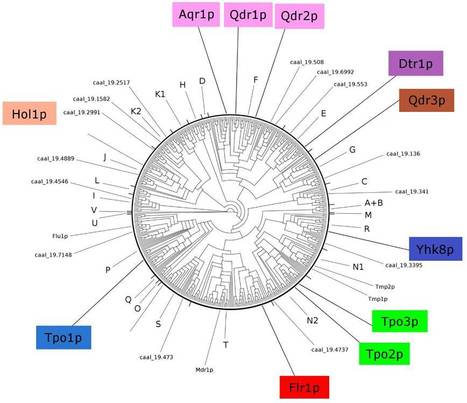
Candida species colonize different body sites in healthy hosts but these yeasts are also opportunistic pathogens when an alteration in human microbial defenses occur. The high mortality rate of Candida bloodstream infections (candidemia) is related to their ability to develop resistance against multiple antifungal agents. In particular, two drug:H+ antiporters of family 1 (DHA1), Mdr1p and Flu1p, are known to underly the azole-resistance phenotype observed in many C. albicans clinical isolates. In a recent paper published on the journal Genomics, iBB researchers of the Biological Sciences Research Group (BSRG) identified the whole set of DHA1 proteins encoded in the genomes of 31 hemiascomycetous strains, corresponding to 25 yeast species. Using phylogenetic tree building methodologies and comparative genomic approaches, the evolutionary history of the DHA1 encoding genes in the Hemiascomycetes was reconstructed, revealing that sixteen DHA1 lineages were conserved during pathogenic Candida species evolution. The evolution of C. albicans MDR1 and FLU1 genes and C. dubliniensis, C. tropicalis and C. parapsilosis MDR1 genes was detailed and gene duplication and loss were found to be major driving forces underlying the evolution of the DHA1 genes in Candida species.

|
Scooped by
iBB
July 19, 2014 6:31 PM
|
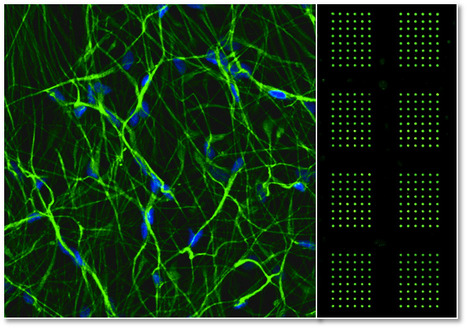
In collaboration with Rensselaer Polytechnic Institute (RPI/USA), BERG researchers at the Stem Cell Bioengineering and Regenerative Medicine Laboratory (SCBL) have recently developed a 3D microarray platform to perform high-throughput studies of human Neural Stem Cell (hNSC) Differentiation and Toxicology. By using this platform it is possible to screen for the differential toxicity of small molecules to hNSCs which may help to predict, in vitro, which compounds pose an increased threat to neural development and should therefore be prioritized for further screening and evaluation. The work was published in “Stem Cell Research” journal. Click on title to learn more,

|
Scooped by
iBB
July 16, 2014 6:25 AM
|
Early this year, the newest version of the YEASTRACT database was released in a paper in Nucleic Acids Research, resulting from the effort of a team of researchers from the BSRG/IBB and the KDBIO/INESC-ID. The YEASTRACT information system has been an important tool for thousands of users from the Yeast and Systems Biology communities for the past decade. Its new version offers unprecedented tools for the analysis and prediction of the regulatory control in budding yeast, at gene and genomic levels. Click on title to learn more. Photo details: Yeast cell membrane visualized with RFP and GFP fluorescent markers, Masur, 2006, GNU Free Documentation license. http://commons.wikimedia.org/wiki/File:Yeast_membrane_proteins.jpg
|

|
Scooped by
iBB
July 20, 2015 1:41 PM
|
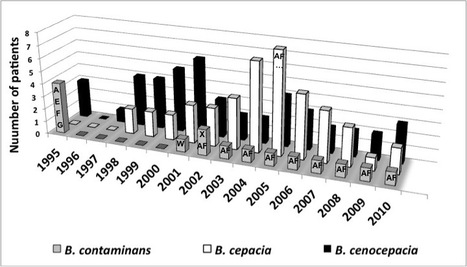
The taxonomy of Burkholderia cepacia complex (Bcc) has recently evolved significantly. The novel species B. contaminans and B. lata were proposed for a divergent group of bacteria formerly classified as B.cepacia recA K. In a recent publication in the Journal of Medical Microbiology, Carla Coutinho and Isabel Sá-Correia from BSRG-iBB, together with researchers from Hospital de Santa Maria (HSM) in Lisbon, have re-examined the taxonomic position, at the species level, of several isolates previously classified as B. cepacia recA K from a Bcc collection and also extended the identification to other isolates retrieved from CF patients under surveillance at the major Portuguese CF Center at HSM. During 15 years of epidemiological surveillance of respiratory infections involving Bcc in this Portuguese CF Center, B. contaminans was found to be involved in chronic and transient infections or to have been eradicated. The clinical outcome of the infected patients under study provided new information on the clinical impact of the rarely found B. contaminans species in CF respiratory infections. Click on tile to learn more.

|
Scooped by
iBB
July 6, 2015 6:14 AM
|
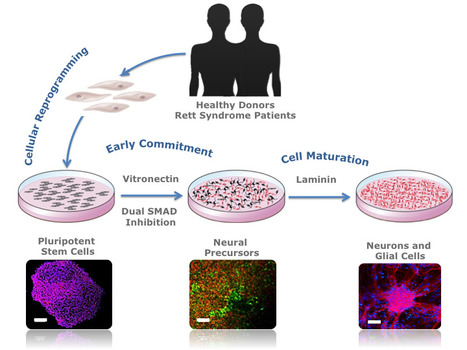
In a recent publication in Biotechnology Journal researchers from the Stem Cell Bioengineering and Regenerative Medicine Laboratory (SCBL) at BERG-iBB describe a novel methodology capable of providing patient-specific neural cells under defined conditions using vitronectin and dual SMAD inhibition. The authors show how pluripotent stem cells can be used to generate patient-specific neural cells that could be used to gain a better understanding of disease mechanisms. This ability to recapitulate the development of the human nervous system in vitro could provide important insights on the mechanisms involved in the maturation of specific neural cell types, making this approach transversal to other related areas in neurodevelopmental research.

|
Scooped by
iBB
June 8, 2015 12:53 PM
|
The quest for renewable fuels from sustainable and ecologically friendly processes has identified biodiesel as a promising candidate. Carla de Carvalho and Maria Cortes of BERG-iBB recently showed that bacterial cells of Rhodococcus erythropolis DCL14 and R. opacus PWD4 can accumulate triacylglycerols, the most relevant lipids for biodiesel production, and other lipids which contain fatty acids that may be esterified. Appropriate growth conditions, such as N and P limitation, increased lipid accumulation in the cells. The cetane numbers of the fatty acid blends produced, which measure the quality of diesel fuels and biodiesel, are in accordance to biodiesel standards.

|
Scooped by
iBB
June 6, 2015 6:21 AM
|
3D suspension culture is generally considered a promising method to achieve efficient expansion and controlled differentiation of human pluripotent stem cells (hPSCs). In a recent publication in Biotechnology Journal, researchers from the Stem Cell Bioengineering and Regenerative Medicine Laboratory (SCBL) at BERG-iBB led by Margarida Diogo describe the development of an integrated culture platform for expansion and neural commitment of hPSCs into neural precursors using 3D suspension conditions and chemically-defined culture media. The results obtained in the study constitute an important contribution to the definition of a robust method for production of hPSC-derived neural precursors that minimizes processing steps and has high potential for scale-up.

|
Scooped by
iBB
April 9, 2015 4:44 PM
|
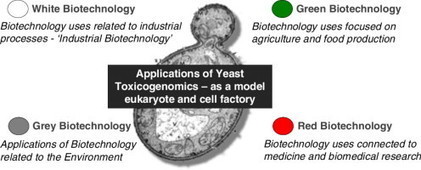
The 2015 edition of the special issue Environmental Biotechnology published by Current Opinion in Biotechnology features a review on the field of yeast toxicogenomics, focusing on the role of this organism both as a cell model and as a cell factory. Toxicogenomics has emerged in the past decade as a transdisciplinary area that merges genome-wide methodologies with traditional toxicology studies to provide an integrated assessment of the cellular response to different toxicants at multiple levels. The invitation to write this review was made on the basis of the longstanding contribution of the Biological Sciences Research Group-iBB and its PI Isabel Sá-Correia to this field.
The still highly relevant role of yeast as a model organism is highlighted in this review, supported by the pioneering exploitation of multiple Omics approaches (genomics, proteomics, metabolomics, transcriptomics, lipidomics...) and synthetic biology, in fields ranging from the pharmaceutical industry to food preservation processes and identification of toxicological outcomes of exposure to environmental toxicants and agrochemicals. The review also describes the most recent advances in the production of next-generation biofuels based on renewable feedstocks such as lignocellulosic hydrolysates. Yeast is a cell factory of choice in biorefineries, where genomic and metabolic engineering are greatly contributing to develop more cost-efficient bioprocess conditions and more robust engineered strains. Click on the title to learn more.

|
Scooped by
iBB
October 18, 2014 10:42 AM
|
A joint publication from BSRG-iBB and IMAR & MARE – University of Coimbra in the journal Science of the Total Environment describes evaluation of the suitability of a short-term yeast-based gene expression assay to assess the toxicity of pyrimethanil in runoff samples simulated from soils sprayed with fungicide accidental spill doses. Comparison of toxicity data to the yeast and to aquatic and soil standard species pointed the yeast assay relevant for screening worst-cases of fungicide contamination. This work was performed in the context of Fátima Gil’s PhD in Biotechnology (finished july 2014; supervisors: Cristina A Viegas, Jorge H Leitão). Photo sources: en.wikipedia.org, www.zmescience.com, www.marietta.edu, enfo.agt.bme.hu, www.infojardin.com

|
Scooped by
iBB
August 30, 2014 4:17 PM
|
For processes to be implemented on an industrial scale, high volumetric productivity and long term stability are required. In a paper recently published in Bioresource Technology, a team headed by Pedro Fernandes (BERG-IBB, IST, ULisboa) and Maria Henriques Ribeiro (iMed, Faculdade de Farmácia, ULisboa) presented a prototype enzyme reactor enabling the hydrolysis of naringin, a reaction that decreases the bitterness of grape juice and other citrus juices, while maintaining their organoleptic and anti-oxidant properties. The reactor operated continuously for over a month with no decrease in catalytic activity and a volumetric productivity of 7 mM/h, thus proving an appealing asset for the food processing industry.

|
Scooped by
iBB
August 15, 2014 10:11 AM
|
In a recent paper published in Applied Microbiology and Biotechnology, researchers from BERG-iBB, MIT, Institut Pasteur, Instituto Politécnico de Setúbal and University of New York unravel new genetic and environmental determinants that shape the transposition rate of type 2 insertion sequences (IS2). These were found to encompass asymmetries in target DNA composition, medium composition, growth-phase, and production scale. The importance of these findings is manifold, going from the design of safer DNA-based therapeutics to the ON/OFF regulation of synthetic genetic circuits. Click on title to learn more. Photo: South Tower at IST, home of iBB labs. http://tecnico.ulisboa.pt/

|
Scooped by
iBB
July 30, 2014 11:30 AM
|
Pyrimethanil is an anilinopyrimidine fungicide mostly applied in vineyards. When good agricultural practices (e.g. recommended doses or safety periods) are not followed, residue levels detected in grape must or in the environment may be of concern. In a paper published jointly with Jörg Becker from IGC in The Journal of Agricultural and Food Chemistry, BSRG researchers led by Cristina Viegas describe a transcriptomic study to identify molecular indicators of pyrimethanil toxicity and response in the eukaryotic model S. cerevisiae, while providing new mechanistic insights with possible relevance in wine yeasts, phytopathogenic fungi and/or non-target biota in ecosystems. Photo: Crimson grapes, Bob. Nichols, USDA 2013, Public domain. https://commons.wikimedia.org/wiki/File:Crimson_seedless_grapes_on_the_vine.jpg?uselang=pt.

|
Scooped by
iBB
July 29, 2014 5:36 AM
|
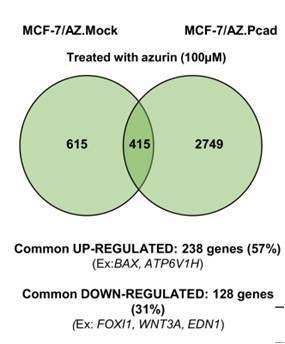
BSRG researchers have unveiled new targets for the anti-cancer protein azurin using a breast cancer model. In a paper published jointly with colleagues from IPATIMUP in The International Journal of Biochemistry and Cell Biology, the team led by Arsénio Fialho describes the use of DNA microarrays to understand the effects of azurin in invasive breast cancer cells overexpressing P-cadherin. The gene transcription data gathered shows that apoptosis is induced and endocytosis is up-regulated to mediate the controlled process of azurin entry in cells. A number of genes coding for membrane receptors frequently overexpressed in breast cancer are down-regulated, suggesting that these new targets could be exploited in the future. Click on title to learn more.

|
Scooped by
iBB
July 23, 2014 5:39 AM
|
Researchers from BERG-iBB and the Prather lab at MIT developed a pgi gene knockout E. coli strain with the specific goal of producing large amounts of plasmid DNA vectors. The new strain was tested by Nature Technology Corporation in the US, yielding 2100-2200 mg plasmid DNA per L in 14 L bioreactors. The strain may be usefull in the large scale production of plasmid-based gene transfer vectors with application in gene therapy, DNA vaccination and recombinant protein production by transient transfection. The research was developed under the framework of the MIT-Portugal program and is a direct result of Geisa Gonçalves (in the photo) Ph D. thesis in Bioengineering. Click on title to learn more.

|
Scooped by
iBB
July 17, 2014 10:57 AM
|
In a recent paper published in Journal of Bionic Engineering, researchers from BERG-IBB studied the topography and wettability of the underside of English weed (Oxalis pes-caprae) leaves using epoxy replicas created via a two-step casting process. Leaves were found to be close to super hydrophobic due to the presence of a characteristic pattern of irregular 100 µm – 200 µm × 60 µm convex papillae. The water repellency properties of such microstructured surfaces may have important applications, including self-cleaning, anti-microbial and anti-fouling. Photo details: SEM image of an epoxy replica of the leaf of English weed. P.M. Pereira, 2013.
|
 Your new post is loading...
Your new post is loading...
 Your new post is loading...
Your new post is loading...