 Your new post is loading...
 Your new post is loading...

|
Scooped by
iBB
May 3, 2023 5:00 AM
|
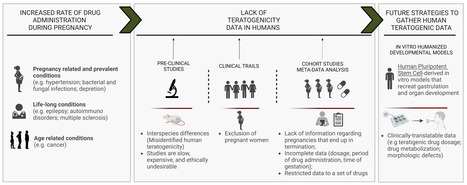
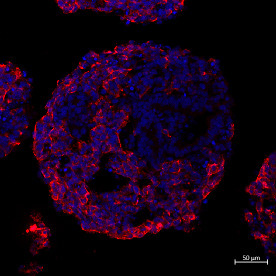
Developmental Toxicity Studies: The Path towards Humanized 3D Stem Cell-Based Models
Today, it is recognized that medicines will eventually be needed during pregnancy, either due to gestation-related medical conditions or pre-existing diseases. Also, the rate of drug prescription to pregnant women has increased over the past few years, in accordance with the increasing trend to postpone childbirth to a later age. However, information regarding teratogenic risk in humans is often missing for most of the purchased drugs. Inter-species differences have limited the suitability of animal models to predict human-specific outcomes, contributing to misidentified human teratogenicity. Therefore, the development of physiologically relevant in vitro humanized models can be the key to surpassing this limitation. In this context, this review describes the pathway towards the introduction of human pluripotent stem cell-derived models in developmental toxicity studies.

|
Scooped by
iBB
November 18, 2022 5:14 AM
|
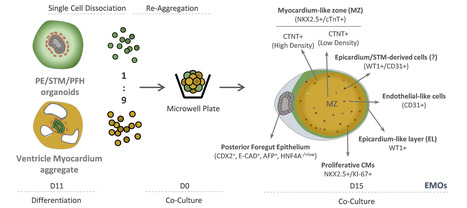
Recreating Human Heart Development in the Lab
The embryonic development of the human heart under healthy and pathological conditions is still very poorly understood. In a recently published Nature Communications paper, a iBB team led by Margarida Diogo, in collaboration with Perpetua Pinto do Ó at i3S, engineered a unique organ-like model from stem cells that recreates several stages of embryonic development of the human heart. This humanized model opens now the path to study, in a tissue engineering lab, the development of congenital heart diseases, such as coronary vascular diseases and myocardium non-compaction cardiomyopathies, and can be further applied in drug screening and toxicology assays during embryonic heart development. This last topic is particularly relevant since pregnant women normally do not take part in clinical trials, making this human cell-based in vitro platform the only viable alternative to assess developmental cardiotoxicity. The work was a part of the PhD thesis in Bioengineering – MIT Portugal program of Mariana Branco, the first author of the paper, and was funded by the FCT project “Mini-Hearts - Organoid Engineering for Production of 3D Cardiovascular Microtissues from Human Induced Pluripotent Stem Cells for Cardiotoxicity”.

|
Scooped by
iBB
December 30, 2020 6:08 AM
|
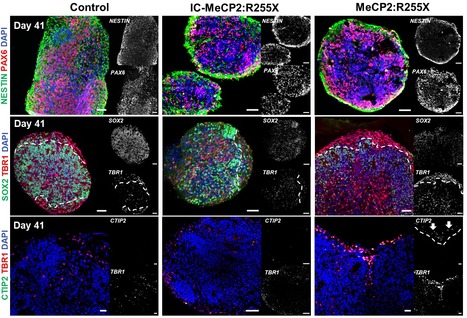
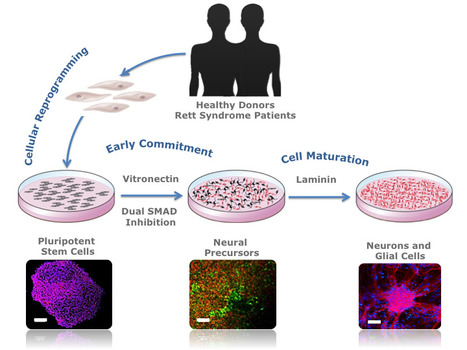
Modeling Rett Syndrome With Human Patient-Specific Forebrain Organoids
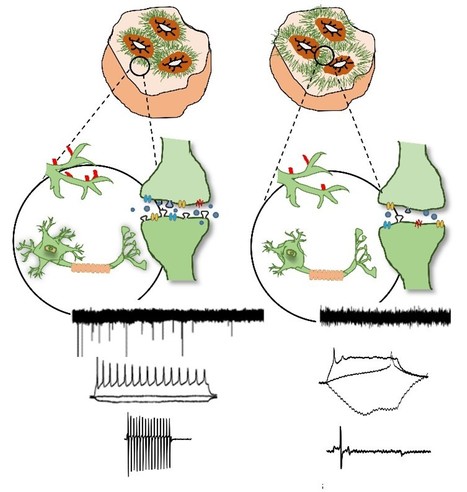
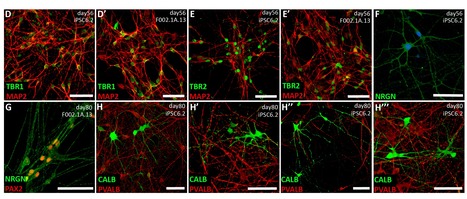
Engineering brain organoids from human induced pluripotent stem cells (hiPSCs) is a powerful tool for modeling brain development and neurological disorders. Rett syndrome (RTT), a rare neurodevelopmental disorder, can greatly benefit from this technology, since it affects multiple neuronal subtypes in forebrain sub-regions. SCERG-iBB researchers have recently established dorsal and ventral forebrain organoids from control and RTT patient-specific hiPSCs recapitulating the 3D organization and functional network complexity of this brain region. The data obtained revealed a premature development of the deep-cortical layer, associated to the formation of TBR1 and CTIP2 neurons, and a lower expression of neural progenitor/proliferative cells in RTT dorsal organoids. Moreover, calcium imaging and electrophysiology analysis demonstrated functional defects of RTT neurons. Additionally, assembly of RTT dorsal and ventral organoids revealed impairments of interneuron’s migration. Overall, these models provide a better understanding of RTT during early stages of neural development, demonstrating a great potential for personalized diagnosis and drug screening. The paper was published in Frontiers in Cell Development Biology.

|
Scooped by
iBB
July 5, 2019 10:05 AM
|
Transcriptomic Analysis of 3D Cardiac Differentiation of Human Induced Pluripotent Stem Cells
Human induced pluripotent stem cells (hiPSCs) represent an almost limitless source of cells for biomedical applications including cardiomyocytes (CMs), the most predominant cell type in the human heart. iBB researchers have established an efficient and robust 3D platform for CM production from hiPSCs and studied the impact of 3D culture on CM differentiation and maturation compared with a 2D monolayer culture. It was found that CMs mature earlier and show an improved communication system in this 3D environment which was suggested to be responsible for a higher structural and functional maturation. This novel 3D culture platform and the CMs obtained can be used for disease modelling, drug screening and cardiotoxicity tests. The results were published in Scientific Reports.

|
Scooped by
iBB
June 21, 2018 12:30 PM
|
Expansion of Human Induced Pluripotent Stem Cells in Vertical-Wheel Bioreactors
The successful use of Human induced Pluripotent Stem Cells (hiPSC) for disease modelling, drug discovery and, ultimately, for regenerative therapies depends on the development of robust bioprocesses capable of generating large numbers of hiPSC and derivatives. SCERG-iBB researchers developed a bioprocess for the scalable generation of hiPSC in a microcarrier-based system using, for the first time, single-use Vertical-Wheel bioreactors. hiPSC culture was performed in working volumes up to 300 mL, maintaining the pluripotency and genomic integrity of the cells, providing an important tool for the successful manufacturing of hiPSC-based products. The work was published in the Journal of Chemical Technology and Biotechnology.

|
Scooped by
iBB
May 25, 2018 7:19 AM
|
Neural Induction of Human Induced Pluripotent Stem Cells for Neurodevelopmental Toxicity Studies
The ability to differentiate neural progenitors (NP) from human induced pluripotent stem cells (hiPSCs) provides an opportunity to develop new applications for cellular therapy, disease modelling and drug screening. SCERG-iBB researchers developed a platform that can be applied towards the study of the effect of neurotoxic molecules that impair normal embryonic development, such as the antiepileptic drug valproic acid (VPA). It was verified that exposure to VPA led to a prevalence of NP structures over neuronal differentiation, confirmed by analysis of the expression of neural cell adhesion molecule, and neural rosette number and morphology. This methodology can potentially complement current toxicity tests for the detection of teratogenic compounds that can interfere with normal embryonic development. The work was published in Toxicology Letters.

|
Scooped by
iBB
November 13, 2017 3:16 PM
|
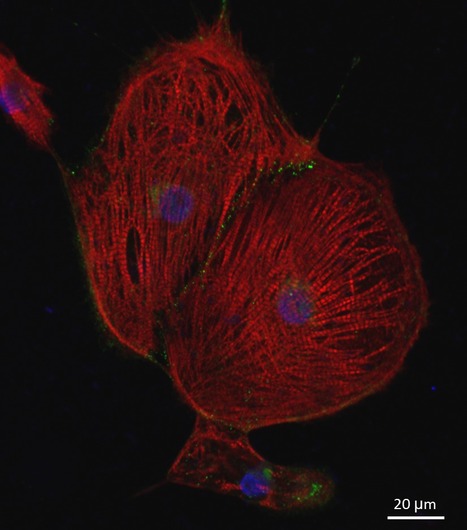
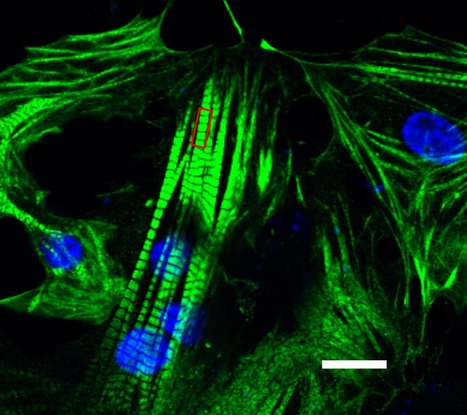
Featured Photo: hiPSC-Derived Cardiomyocytes
Description: Culture of hiPSC-derived cardiomyocytes expressing Cardiac Troponin T (green) and N-Cadherin (red) featured photo by Tiago Dias, Copyright SCERG-iBB 2016.
Context: SCERG researchers led by Joaquim Cabral, Margarida Diogo and Tiago Fernandes are establishing new methodologies for cardiac differentiation of human induced pluripotent stem cells (hiPSC). The work is being performed in the context of iBB’s Strategic Area 1: Stem Cell Engineering.

|
Scooped by
iBB
May 9, 2017 2:09 PM
|
Gonçalo Rodrigues will be defending his Ph D thesis in Biotechnology and Biosciences at Instituto Superior Técnico, thursday the 11th may 2017 (10:30 H, room PA3). During the last years, and under the supervision of Joaquim Cabral and Margarida Diogo from SCERG-iBB and David V. Schaffer from the University of California, Gonçalo focused his efforts on the study of neural differentiation for cell therapy applications. The title of Gonçalo's thesis is “Defined and Scalable Systems for Neural Differentiation of Human Pluripotent Stem Cells”.

|
Scooped by
iBB
May 5, 2016 12:16 PM
|
Tiago Dias will be defending his Ph D thesis in Biotechnology and Biosciences at Instituto Superior Técnico, tuesday the 10th may 2016 (15:00 H, room PA3). During the last years, and under the supervision of Joaquim Cabral and Margarida Diogo from BERG-IBB, Tiago focused his efforts on the study of the signaling behind the differentiation of stem cells into neuroectoderm, mesoderm (cardiomyocytes) and endoderm. The title of Tiago's thesis is “Human Pluripotent Stem Cell Signaling and Cardiac Lineage Commitment”.

|
Scooped by
iBB
June 6, 2015 6:21 AM
|
3D suspension culture is generally considered a promising method to achieve efficient expansion and controlled differentiation of human pluripotent stem cells (hPSCs). In a recent publication in Biotechnology Journal, researchers from the Stem Cell Bioengineering and Regenerative Medicine Laboratory (SCBL) at BERG-iBB led by Margarida Diogo describe the development of an integrated culture platform for expansion and neural commitment of hPSCs into neural precursors using 3D suspension conditions and chemically-defined culture media. The results obtained in the study constitute an important contribution to the definition of a robust method for production of hPSC-derived neural precursors that minimizes processing steps and has high potential for scale-up.
|

|
Scooped by
iBB
January 19, 2023 2:09 PM
|
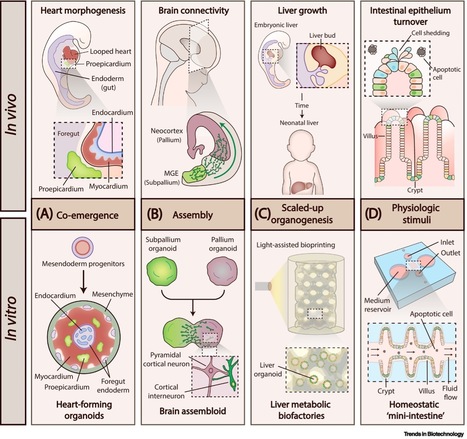
Advancing organoid design through co-emergence, assembly, and bioengineering
Human adult stem cells and patient-derived induced pluripotent stem cells represent promising tools to understand human biology, development, and disease. Under a permissive environment, stem cell derivatives can self-organize and reconstruct their native environment, resulting in the creation of organ-like entities known as organoids. Although organoids represent a breakthrough in the stem cell field, there are still considerable shortcomings preventing their widespread use, namely their variability, limited function, and reductionist size. In this recently published review article in Trends in Biotechnology, Miguel Tenreiro (Ph.D. student in Bioengineering), Prof. Margarida Diogo, and co-authors from iBB highlight emerging technologies to fill in the gaps in organoid design and provide insights on how they can be best utilized to fulfill the potential of organoids.

|
Scooped by
iBB
April 30, 2021 6:28 AM
|
Modeling Rett Syndrome with Human Pluripotent Stem Cells: Mechanistic Outcomes and Future Clinical Perspectives
Rett syndrome (RTT) is a rare neurodevelopmental disorder caused by mutations in the gene encoding for the MeCP2 protein. Among different roles, MeCP2 has a high phenotypic impact during the different stages of brain development. Thus, it is essential to investigate the function of MeCP2 and its regulated targets. In a review paper published in the International Journal of Molecular Sciences, a team of researchers at SCERG-iBB provides a brief summary of the main neurological features of RTT and of the impact of MeCP2 mutations in the neuropathophysiology of the disease. A thorough revision of recent advances and future prospects of RTT modeling using human neural cells derived from pluripotent stem cells and its contribution for the current and future clinical trials for RTT is also provided.

|
Scooped by
iBB
June 17, 2020 12:32 PM
|
Maturation of Human Pluripotent Stem Cell-Derived Cerebellar Neurons in the Absence of Co-Culture
In a new paper published in Frontiers in Bioengineering and Biotechnology, SCERG-iBB researchers in collaboration with colleagues from the Institute of Molecular Medicine (iMM) describe a novel differentiation strategy that uses defined medium to generate Purkinje cells, granule cells, interneurons, and deep cerebellar nuclei projection neurons, that self-formed and matured into electrically active cells. This research is expected to result in better models for the study of cerebellar dysfunctions and represent an important advancement towards the development of autologous replacement strategies for treating cerebellar degenerative diseases.

|
Scooped by
iBB
October 31, 2018 10:10 AM
|
Expansion and Harvesting Of Human Induced Pluripotent Stem Cells on Dissolvable Microcarriers
Development of efficient bioprocesses for human induced pluripotent stem cells (hiPSC) is critical for their medical and biotechnological applications. Scalable expansion of hiPSC is often performed using polystyrene microcarriers, which have to be removed using a time-consuming separation step. At the Stem Cell Engineering Research Group, novel xeno-free dissolvable microcarriers were applied for the first time for the integrated expansion and harvesting of hiPSC. After expansion, microcarriers were dissolved inside the bioreactor, allowing the recovery of more than 90% of the cells, which represents a significantly higher cell yield when compared with microcarrier filtration (45%). These results represent a major improvement for the downstream processing of hiPSC. Find more on the paper on Biotechnology Journal.

|
Scooped by
iBB
June 4, 2018 10:11 AM
|
Engineeering of Mini-Hearts
The project "Mini-Hearts: Organoid Engineering for Production of 3D Cardiovascular Microtissues from Human Induced Pluripotent Stem Cells for Cardiotoxicity" has been recommended for funding by FCT (2017 Call for SR&TD Project Grants). The goal of "Mini-Hearts is to develop an innovative model of cardiovascular tissue from human induced pluripotent stem cells (hiPSC) for drug screening, toxicology assays, disease modeling and potentially for Regenerative Medicine applications. The work will explore the new paradigm of organoid engineering by targeting the simultaneous differentiation of hiPSCs into all the cell types of the human myocardium. This will potentially allow the reproduction of the heterogeneity, complexity, maturity and functionality of cardiovascular tissue. The project, which falls within the scientific area of Medical Engineering, is headed by Margarida Diogo from SCERG-iBB.

|
Scooped by
iBB
May 18, 2018 11:36 AM
|
Structural Maturation of Human Cardiomyocytes Derived from Pluripotent Stem Cells
Cardiomyocytes derived from human induced pluripotent stem cells (hiPSC-CMs) have an enormous potential for in vitro modeling of cardiac diseases and for testing the effect and toxicity of new drugs. However, when compared to adult CMs, hiPSC-CMs are very immature, exhibiting low structural development and functionality. SCERG-iBB researchers developed a novel methodological framework to quantify structural aspects of hiPSC-CMs during long-term culture. hiPSC-CMs showed significant progression in several structural characteristics namely cardiomyocyte fiber density and length. Importantly, this methodology contributes to set new metrics to develop applications for drug screening and disease modeling for hiPSC-CMs. The research has been published on Biochemical and Biophysical Research Communications.

|
Scooped by
iBB
June 9, 2017 5:30 AM
|
Oligodendrocyte precursor cells (OPCs) offer considerable potential for the treatment of demyelinating diseases and injuries of the CNS. However, generating large quantities of high-quality OPCs remains a challenge that impedes their therapeutic application. In collaboration with researchers at the Stem Cell Engineering Research Group (SCERG), researchers at the Department of Bioengineering, University of California, Berkeley, have recently developed a novel defined and scalable culture system for differentiation of OPCs from Human Pluripotent Stem Cells, under 3D conditions, using a fully-defined thermoresponsive biomaterial. This system enabled the generation of large quantities of high-quality OPCs that were able to engraft, migrate and mature into myelinating oligodendrocytes upon transplantation into the brains of NOD/SCID mice.

|
Scooped by
iBB
July 21, 2016 11:41 AM
|
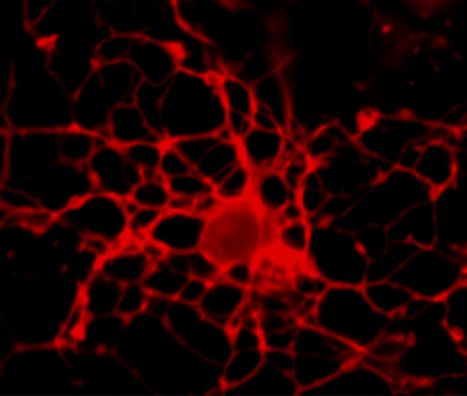
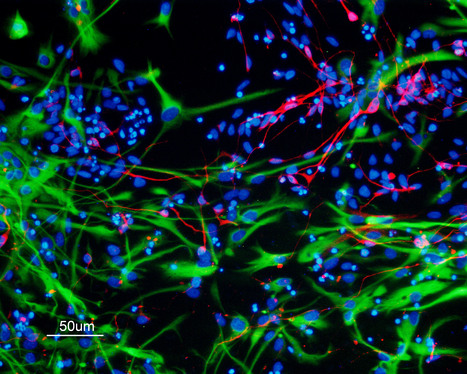
Featured Photo: hiPSC-Derived Astrocytes and Neurons
Description: Culture of hiPSC-derived astrocytes (green) and neurons (red), featured photo by Cláudia Miranda, Copyright BERG-iBB 2016.
Context: BERG researchers led by Joaquim Cabral, Margarida Diogo and Tiago Fernandes are developing new methodologies for neural differentiation of human induced pluripotent stem cells (hiPSC). The work is being performed in the context of iBB’s Strategic Area 1: Stem Cell Engineering.

|
Scooped by
iBB
July 6, 2015 6:14 AM
|
In a recent publication in Biotechnology Journal researchers from the Stem Cell Bioengineering and Regenerative Medicine Laboratory (SCBL) at BERG-iBB describe a novel methodology capable of providing patient-specific neural cells under defined conditions using vitronectin and dual SMAD inhibition. The authors show how pluripotent stem cells can be used to generate patient-specific neural cells that could be used to gain a better understanding of disease mechanisms. This ability to recapitulate the development of the human nervous system in vitro could provide important insights on the mechanisms involved in the maturation of specific neural cell types, making this approach transversal to other related areas in neurodevelopmental research.

|
Scooped by
iBB
July 19, 2014 6:31 PM
|
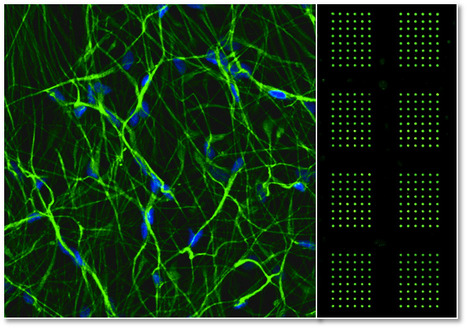
In collaboration with Rensselaer Polytechnic Institute (RPI/USA), BERG researchers at the Stem Cell Bioengineering and Regenerative Medicine Laboratory (SCBL) have recently developed a 3D microarray platform to perform high-throughput studies of human Neural Stem Cell (hNSC) Differentiation and Toxicology. By using this platform it is possible to screen for the differential toxicity of small molecules to hNSCs which may help to predict, in vitro, which compounds pose an increased threat to neural development and should therefore be prioritized for further screening and evaluation. The work was published in “Stem Cell Research” journal. Click on title to learn more,
|



 Your new post is loading...
Your new post is loading...



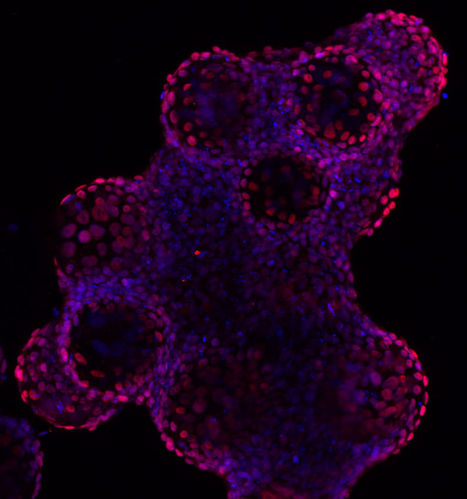
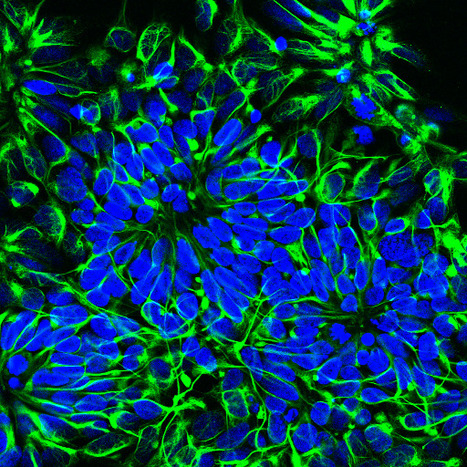
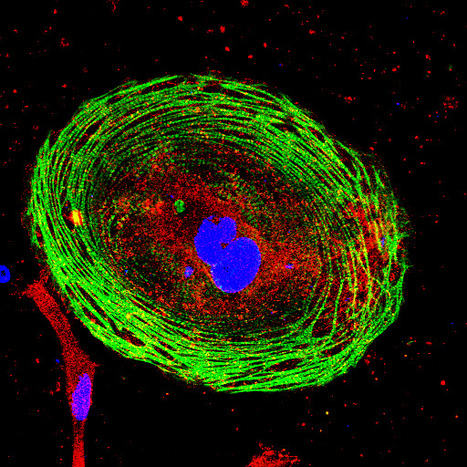


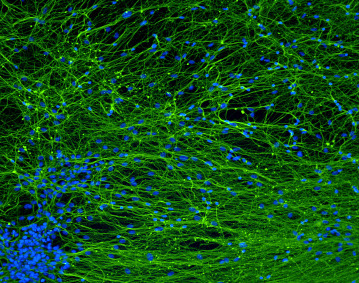





Check full paper here.