 Your new post is loading...
 Your new post is loading...

|
Scooped by
iBB
July 11, 2023 5:59 AM
|
Extracellular Vesicles and Infection
Extracellular vesicles (EVs) are small membrane bound structures released by cells into the extracellular space. They have been shown to transport different molecules such as proteins, nucleic acids and lipids to other cells, serving as vehicles of intracellular communication. EVs have also been shown to play important roles during viral and bacterial infection. Viruses can hijack the biogenesis of EVs to promote viral spreading. Additionally, these EVs are also important mediators in inflammation and immune responses during both bacterial and viral infections. A recent publication in the journal Pharmaceutics, by Diogo Gonçalves, Sandra Pinto and Fábio Fernandes (from iBB/IST), reviews these mechanisms and described the impact of bacterial EVs in regulating immune. Finally, the review also focuses on the potential and challenges of using EVs to tackle infectious diseases.

|
Scooped by
iBB
January 31, 2023 8:46 AM
|
Choosing the right fluorescent probe
Fluorescence microscopy and spectroscopy are routine tools in basic and applied biological sciences. An expanding library of organic fluorophores and fluorescent proteins with radically different properties are available, offering great flexibility to the user of fluorescence methods. Still, a considerable fraction of casual users of fluorescence tools do not follow rational considerations when selecting a probe. In a recently published book chapter, Fábio Fernandes (iBB) and Maria Sarmento (IMM) provide an overview of the most important factors to weight when selecting a fluorophore. A list of different fluorophores and a summary of their properties and labeling strategies is presented as a tool to assist in the process of choosing a fluorescent probe.

|
Scooped by
iBB
November 26, 2021 11:26 AM
|
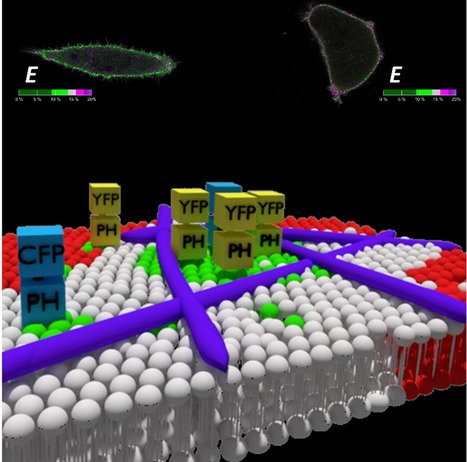
Quantitative FRET Microscopy Reveals a Crucial Role of Cytoskeleton in Promoting PI(4,5)P2 Confinement
Phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) is crucial to many cellular processes in eukaryotes, including membrane trafficking, signal transduction, ion channel function and cytoskeleton dynamics. This function multiplicity is partially achieved through a dynamic spatiotemporal organization of PI(4,5)P2 within the membrane. In a recent paper published in IJMS, an IBB team (Maria J. Sarmento, Luís Borges-Araújo, Sandra N.Pinto, Nuno Bernardes, Joana Ricardo, Ana Coutinho, Manuel Prieto and Fábio Fernandes) was able to quantify PI(4,5)P2 confinement in living cells making use of FRET imaging measurements. PI(4,5)P2 was found to be significantly compartmentalized at the plasma membrane of HeLa cells. These PI(4,5)P2 enriched domains were shown to not depend on cholesterol content, ruling out an association with lipid rafts. On the other hand, upon inhibition of actin polymerization, compartmentalization of PI(4,5)P2 was almost entirely eliminated, confirming that the cytoskeleton network is the critical component responsible for the formation of nanoscale PI(4,5)P2 domains.

|
Scooped by
iBB
September 3, 2021 10:37 AM
|
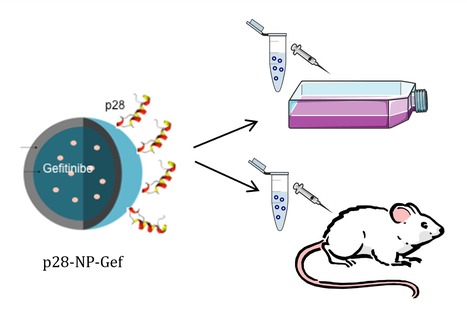
Gefitinib-Loaded p28-PLGA Nanoparticles Reduce Tumor Burden and Metastases in Lung Cancer
p28 is a 28 amino acids peptide derived from the bacterial protein azurin. It possesses cell-penetrating capabilities showing preferential enter in cancer cells. Moreover it has been subject in US to two phase I clinical trials as a anticancer agent. In a recent paper published in Journal of Controlled Release, a iBB team (Garizo AR, Dias TP, Fernandes F, Bernardes N, Fialho AM) together with a i3S/UP team (Castro F, Martins C, Almeida A, Barrias CC, Sarmento B) were able for the first time to fabricate p28-functionalized PLGA nanoparticles (NPs) loaded with the EGFR tyrosine kinase inhibitor gefitinib. The results obtained indicate that these NPs interact preferentially with lung cancer cells due to their decoration with p28 peptide. In vitro cytotoxicity assays demonstrate biological activity of the NPs against lung cancer cancer cells. Finally, in vivo studies demonstrated a great potential of the p28-NPs in enhancing the therapeutic effects of gefitinib.

|
Scooped by
iBB
September 11, 2020 9:17 AM
|
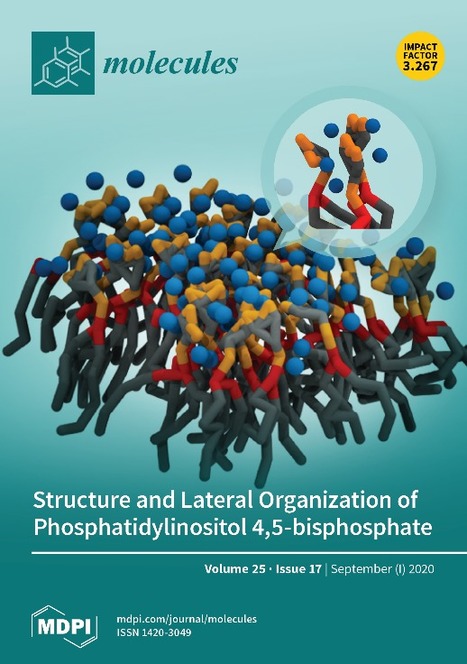
Structure and Lateral Organization of Phosphatidylinositol 4,5- bisphosphate (Cover of Molecules)
Phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) stands out from other plasma membrane lipids as one of the most important regulators of membrane-associated signaling events. PI(4,5)P2 is able to engage in a multitude of simultaneous cellular functions that are temporally and spatially regulated through the presence of localized transient pools of PI(4,5)P2 in the membrane. These pools are crucial for the recruitment, activation, and organization of signaling proteins and consequent regulation of downstream signaling. A review published by the PhD student Luís Borges-Araújo (from the Medical Biochemistry and Biophysics Doctoral Program) and Fábio Fernandes showcases some of the most important PI(4,5)P2 molecular and biophysical properties, as well as their impact on its membrane dynamics, lateral organization, and interactions with other biochemical partners. The review was published in the Molecules Journal and was selected as the cover of the September issue.

|
Scooped by
iBB
July 17, 2018 12:24 PM
|
Azurin Perturbs Lipid Rafts’ Organization and Enhances Sensitivity to Anti-cancer Drugs
A new determinant of the interaction of the bacterial anti-cancer protein azurin with the lipid rafts present in the membrane of cancer cells has been uncovered by a team led by Arsénio Fialho from BSRG-iBB, in collaboration with Sandra Pinto and Fábio Fernandes from CQFM-IN and iBB. The lipid rafts in these cells contribute to increase membrane order, rigidity and resistance to anti-cancer drugs. As a consequence of the interaction with lipid rafts, we demonstrate that treating cells with azurin increases membrane fluidity, and ultimately benefits the action of other drugs, probably by facilitating its entry in cancer cells. The work was recently published in the journal Cell Cycle.
|

|
Scooped by
iBB
February 9, 2023 6:29 AM
|
Initiative kick off of the National Advanced Microscopy Network for Health and Life Sciences, CryoEM-PT
The CryoEM-PT - National Advanced Electron Microscopy Network for Health and Life Sciences facility inauguration was held in February 2nd at the INL - International Iberian Nanotechnology Laboratory, in Braga. The inauguration took place in the presence of Interim INL Director General - Paulo Freitas, the Scientific Coordinator of AEMIS Facility - Paulo Ferreira, the Alderwoman of Braga - Olga Pereira, the FCT President - Madalena Alves and CCDR-N President - António Cunha, along with the representatives of the multiple nodes of this network. Profs. Berberan Santos (iBB President), Fábio Fernandes (IST Node) and Manuel Prieto (iBB) attended the inauguration. IST is one of the nodes of the network and iBB is the authorized user of the equipment on behalf of IST within this network.

|
Scooped by
iBB
December 2, 2021 9:24 AM
|
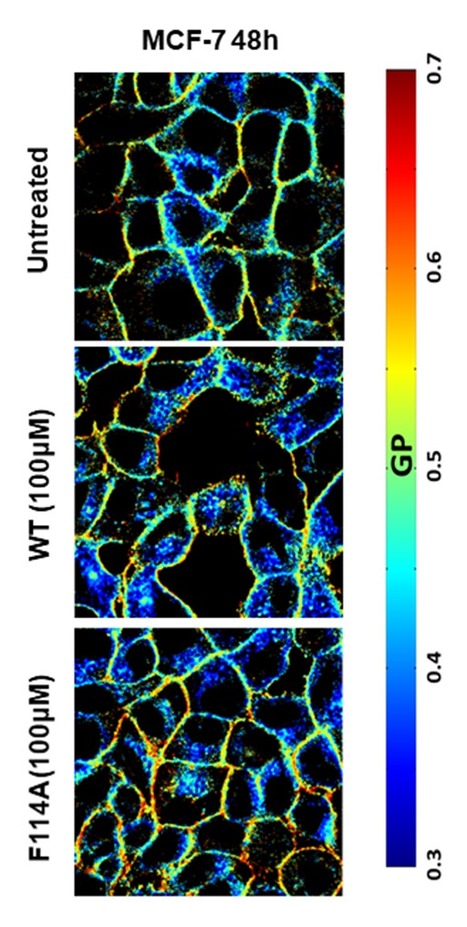
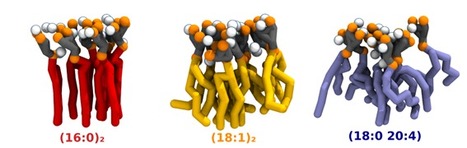
Acyl-chain Saturation Regulates the Order of Phosphatidylinositol 4,5-bisphosphate Nanodomains
PI(4,5)P2 is a phospholipid found mostly in the plasma membrane of eukaryotic cells, where it plays a crucial role in processes like vesicle trafficking, cytoskeletal regulation, ion channel function, viral assembly and budding. While most phospholipids show considerable acyl-chain diversity, PI(4,5)P2 lipids are exceptionally enriched in specific acyl-chains, the most frequent composition in mammalian cells being 1-stearoyl-2-arachidonyl (18:0 20:4). The biological functions that call for this specific enrichment are still not fully clear. In a recent paper published in Communications Chemistry, a BSIRG-iBB team led by Fábio Fernandes together with the teams of Dr. Nuno Santos (IMM) and Dr. Manuel Melo (ITQB) identified a previously unreported increase in membrane order upon calcium-dependent PI(4,5)P2 clustering. Remarkably, the interaction of saturated PI(4,5)P2 with calcium culminated in the formation of gel nanodomains for fully saturated PI(4,5)P2, and the formation of these gel domains was abrogated in the presence of 18:0 20:4 polyunsaturated PI(4,5)P2. These results support a role of (18:0 20:4)PI(4,5)P2 in inhibiting the formation of highly ordered PI(4,5)P2 nanodomains in the plasma membrane.

|
Scooped by
iBB
October 20, 2021 6:08 AM
|
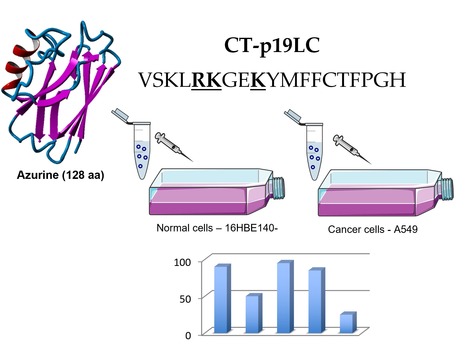
The Azurin-Derived Peptide CT-p19LC Exhibits Membrane-Active Properties and Induces Cancer Cell Death
The bacterial protein azurin shows an unexpected therapeutic effect against various types of cancer. This property seems to result from its unique structural and surface features. A 28-residue peptide (named p28) derived from the middle part of azurin has been subjected to various studies and reached two clinical trials phase I in US. In a recent paper published in Biomedicines, a iBB team (Ana Rita Garizo, Lígia Coelho, Sandra Pinto, Tiago Dias, Fábio Fernandes, Nuno Bernardes and Arsénio M Fialho) were able to identified another anticancer bioactive peptide (CT-p19LC) derived from the C-terminal of azurin. CT-p19LC proved to interact preferentially with cancer cells, causing a significative inhibition of cell proliferation in a dose dependent manner. Moreover, it is proposed that the mode of action of CT-p19LC involves perturbation or disruption of cancer cell membranes. Overall this study highlights the relevance of azurin as a source of bioactive peptides with potential application in cancer therapies.

|
Scooped by
iBB
June 17, 2021 11:40 AM
|
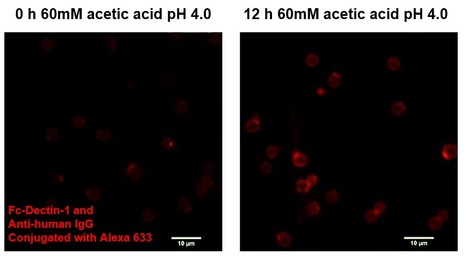
Adaptation to Acetic Acid Stress Involves Structural Alterations and Increased Stiffness of the Yeast Cell Wall
The role of the cell wall in yeast response and tolerance to stress is frequently neglected. A BSRG-iBB research paper just published in Scientific Reports, provides, for the first time, a comprehensive view of the alterations occurring at the cell wall in a yeast population adapting to sub-lethal stress induced by acetic acid. The results reveal changes to the cell wall polysaccharide composition and nanomechanical properties, as well as alterations in the transcript levels of key cell wall biosynthetic genes. This paper reinforces the notion that the adaptive yeast response to acetic acid involves coordinated alterations of the cell wall at the biophysical and molecular levels. The gathered knowledge is important for the design of superior industrial strains and for the efficient control of the deleterious activity of spoilage yeasts, particularly in the Food Industry. This research work is first-authored by the PhD student of the PhD programme in Biotechnology and Biosciences Ricardo Ribeiro (FCT_DP AEM fellowship), performed under the supervision of Isabel Sá-Correia. This collaborative study with Fábio Fernandes (BSIRG-iBB) and Mário S. Rodrigues and his team (BioISI, Faculty of Sciences, ULisboa), is also coauthored by Cláudia Godinho (posdoc researcher) and the PhD student Nuno Bourbon-Melo (FCT_DP BIOTECnico) from the BSRG-iBB team.

|
Scooped by
iBB
May 15, 2019 8:52 AM
|
Lateral and Septal Peptidoglycan Synthesis in Staphylococcus aureus
For many years coccus bacteria were thought to lack the elongation machinery present in rod cells. In a recent paper published in Nature Microbiology, Fábio Fernandes from BSIRG-IBB has collaborated with the group of Mariana Pinho from ITQB NOVA, to show that Staphylococcus aureus cells are equipped with some of the same mechanisms that control elongation in rods, mediated by the SEDS protein RodA. However, while in rods RodA is responsible for making truly elongated cells, in cocci it is used for the brief elongation required before and after cell division. More specifically, the authors conclude that the RodA–PBP3 and FtsW–PBP1 pairs mediate sidewall and septal PGN incorporation, respectively, and that their activity must be balanced to maintain coccoid morphology.

|
Scooped by
iBB
May 29, 2018 1:18 PM
|
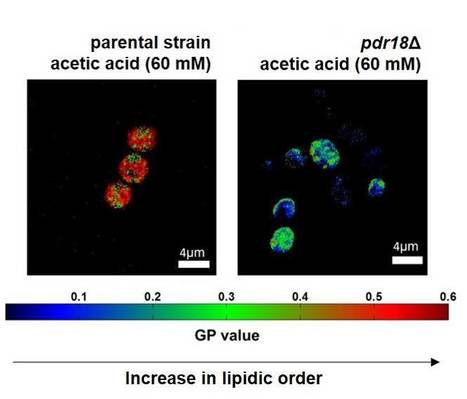
Yeast Response to Acetic Acid Involves Pdr18-mediated Ergosterol Transport at the Membrane
The ability of Saccharomyces cerevisiae to overcome the stress induced by cytotoxic compounds depends on the activity of plasma membrane transporters of the ABC superfamily, presumably through the questionable unspecific efflux of multiple drugs and xenobiotic compounds. A recent paper by iBB researchers provides new insights into the biological role of the ABC transporter of the pleiotropic drug resistance family of putative drug efflux pumps Pdr18, proposed to mediate ergosterol incorporation in plasma membrane. Pdr18 expression was found to help cells to counteract acetic acid-induced decrease of plasma membrane lipid order, increase the non-specific membrane permeability and decrease the transmembrane electrochemical potential. Results support the notion that Pdr18-mediated multistress resistance is linked to the status of plasma membrane lipid environment related with ergosterol content and the associated plasma membrane properties. The paper, published in Scientific Reports, results from the PhD project of Cláudia Godinho, advised by Prof. Isabel Sá-Correia from BSRG-iBB in collaboration with Fábio Fernandes and Sandra Pinto from BSIRG-iBB).
|




 Your new post is loading...
Your new post is loading...


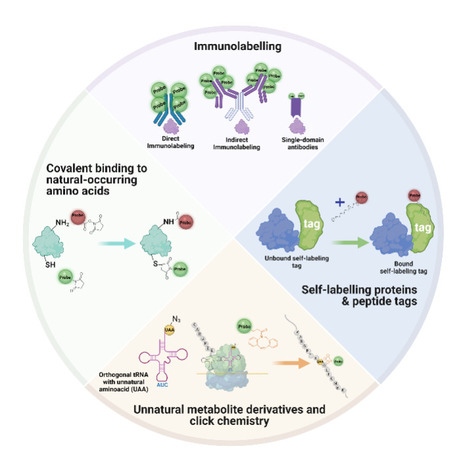







Check full paper here