Over the last couple years, FDA has clarified the scope of its regulation over mobile health. In the agency’s September 2013 guidance, FDA spelled out its oversight for some of the most common mobile medical apps. Then, last month, in two separate draft guidances FDA explained the limits on its oversight of apps used for general wellness like fitness trackers, as well as apps that may be accessories to a medical device. And already in February, FDA published a final guidance deregulatingmedical device data systems, a category that includes numerous mobile apps. The agency has been busy.
But one area FDA has yet to clarify is apps that guide the appropriate use of drugs. Many of those apps potentially fit the category of clinical decision support (CDS) software, an area that FDA has been planning guidance since 2011, but so far has not addressed.
To be fair, this is undoubtedly one of the most difficult guidances to write. Frankly, the number of use cases for CDS is mind-boggling. Compounding matters, creative people are coming up with new use cases on almost a daily basis. For policymakers this is both exciting and challenging.
To help pharmaceutical companies during this period while FDA drafts its proposed guidance, I’d like to summarize what we already know from previous FDA communications, and also offer some thoughts on what the upcoming guidance should address.
Via Pharma Guy



 Your new post is loading...
Your new post is loading...

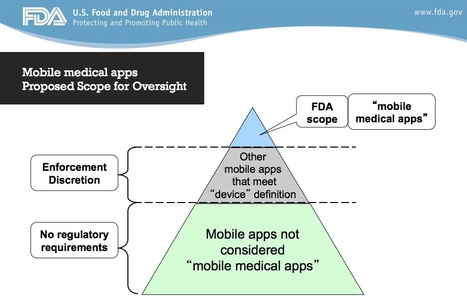






FDA's "Decision Algorithm for General Wellness Products" is pretty incomprehensible! See here.
Meanwhile, I invite you to listen to my interview of Bradley Merrill Thompson in this podcast: Beyond Mobile Medical App Guidance - What to Worry About After FDA Publishes Its "Final" Guidelines.
Talks about how apps for drug use, purchase, etc. will be regulated, but there isn't yet guidance like the ones for health and wellness.