An estimated 128,000 hospitalized patients die each year from Adverse Drug Reactions (ADRs. aka Adverse Events, AEs), which matches stroke as the 4th leading cause of hospital deaths (see here).
Deaths and serious reactions outside of hospitals would signicantly increase the totals. This does not include deaths and hospitalizations from over-dosing, errors, or recreational drug use. We know this because of hundreds of thousands of drug "Adverse Event Reports" (AERs) received by the FDA every year directly from healthcare professionals (HCPs such as physicians, pharmacists, nurses and others), consumers (such as patients, family members, lawyers and others), and drug companies, which are normally required to send AERs it receives from HCPs to the FDA.
The chart above shows the trend in AERs received by the FDA from 2004 through 2015.
I have analyzed data from 2003 through 2014 to look at the number of AERs submitted by HCPs versus consumers, the number of serious adverse events versus the number of adverse events involving death, and the correlation between serious AEs and user fees paid to the FDA by drug companies. I see some interesting trends in the data.
Via Pharma Guy



 Your new post is loading...
Your new post is loading...

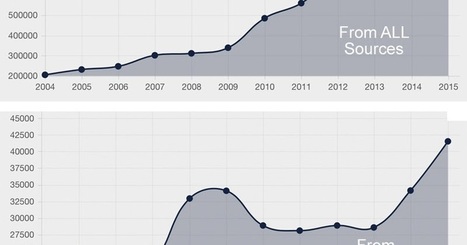







What's responsible for the sharp rise in AERs submitted to FDA by consumers? Could it be class action lawsuits and attorney commercials about the deadly side effects of drugs? Read “AMA Chastises Lawyers for Alarming Drug Ads”; http://sco.lt/5RkA4X