MT @NatRevClinOncol A shed NKG2D ligand that promotes NK cell activation & #tumor rejection http://t.co/fjxcQRyDGS #immunotherapy #cancer
Abstract
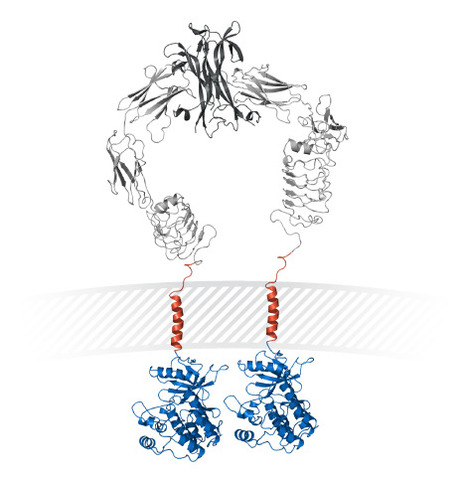
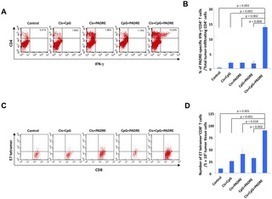
Immune cells, including natural killer (NK) cells, recognize transformed cells and eliminate them in a process termed immunosurveillance. It is thought that tumor cells evade immunosurveillance by shedding membrane ligands that bind to the NKG2D activating receptor on NK cells and/or T cells, and desensitize these cells. In contrast, we show that in mice, shedding of MULT1, a high affinity NKG2D ligand, causes NK cell activation and tumor rejection. Recombinant soluble MULT1 stimulated tumor rejection in mice. Soluble MULT1 functions, at least in part, by competitively reversing a global desensitization of NK cells imposed by engagement of membrane NKG2D ligands on tumor-associated cells, such as myeloid cells. The results overturn conventional wisdom that soluble ligands are inhibitory, and suggest a new approach for cancer immunotherapy.
Via Krishan Maggon


 Your new post is loading...
Your new post is loading...








< Science Express Index Read Full Text to Comment (0)
Science DOI: 10.1126/science.1258867REPORT
ANTITUMOR IMMUNITY
A shed NKG2D ligand that promotes natural killer cell activation and tumor rejectionWeiwen Deng1, Benjamin G. Gowen1, Li Zhang1, Lin Wang1, Stephanie Lau1, Alexandre Iannello1, Jianfeng Xu1,Tihana L. Rovis2, Na Xiong3, David H. Raulet1,*+Author Affiliations
1Department of Molecular and Cell Biology, and Cancer Research Laboratory, University of California at Berkeley, Berkeley, CA, 94720, USA.2Center for Proteomics University of Rijeka Faculty of Medicine Brace Branchetta 20, 51000 Rijeka, Croatia.3Department of Veterinary and Biomedical Sciences, Pennsylvania State University, 115 Henning Bldg, University Park, PA 16802, USA.↵*Corresponding author. E-mail: raulet@berkeley.edu