CAMBRIDGE, Mass., Jun 12, 2015 (BUSINESS WIRE) -- Alnylam Pharmaceuticals, Inc.ALNY, -1.85% a leading RNAi therapeutics company, today announced initial positive results from its ongoing Phase 1/2 clinical trial with ALN-CC5, an investigational RNAi therapeutic targeting complement component C5 for the treatment of complement-mediated diseases. These new clinical data are being presented at the 20th Congress of the European Hematology Association (EHA) held June 11 – 14 in Vienna, Austria. Initial study results from 12 healthy volunteer subjects showed that single subcutaneous dose administration of ALN-CC5 resulted in potent, dose-dependent, durable, and statistically significant knockdown of serum C5 of up to 96%. In addition, single dose administration of ALN-CC5 achieved inhibition of serum complement activity of up to 92%, including an up to 61% inhibition of serum hemolytic activity. Further, ALN-CC5 has been found to be generally well tolerated to date. The company is continuing to dose healthy volunteer subjects in both the single ascending dose (SAD) and multiple ascending dose (MAD) stages of the study, and expects to present additional data from the trial in late 2015. Consistent with previous guidance, the company also plans to begin enrolling patients with paroxysmal nocturnal hemoglobinuria (PNH) into the trial by the end of 2015.
Via Krishan Maggon


 Your new post is loading...
Your new post is loading...








In Ongoing Phase 1/2 Study, Single Subcutaneous Doses of ALN-CC5 Achieve Potent, Statistically Significant, and Highly Durable C5 Knockdown of up to 96% – – After Single Dose, ALN-CC5 Also Achieves up to 92% Inhibition of Serum Complement Activity, Including up to 61% Inhibition of Serum Hemolytic Activity, and was Generally Well Tolerated – – Dose Escalation Continues in Multi-Dose Cohorts, with Additional Data Expected to be Presented in Late 2015
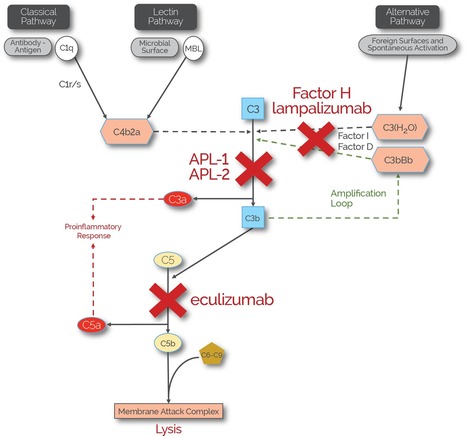
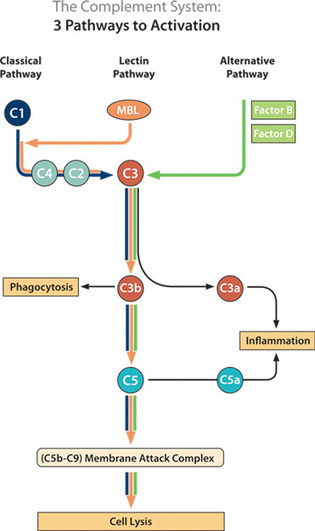
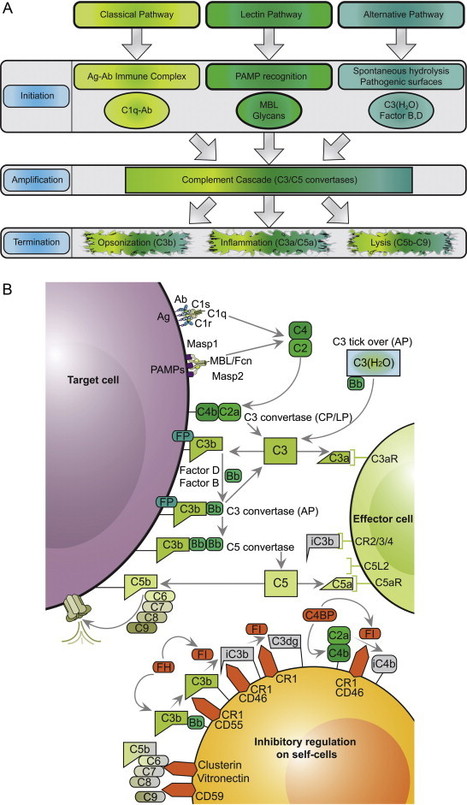
ALN-CC5 is an investigational RNAi therapeutic targeting the C5 component of the complement pathway for the treatment of complement-mediated diseases. The complement system plays a central role in immunity as a protective mechanism for host defense, but its dysregulation results in life-threatening complications in a broad range of human diseases including paroxysmal nocturnal hemoglobinuria (PNH), atypical hemolytic-uremic syndrome (aHUS), myasthenia gravis, neuromyelitis optica, membranous nephropathy, amongst others. Complement component C5, which is predominantly expressed in liver cells, is a genetically and clinically validated target; loss of function human mutations are associated with an attenuated immune response against certain infections and intravenous anti-C5 monoclonal antibody (mAb) therapy has demonstrated clinical activity and tolerability in a number of complement-mediated diseases. A subcutaneously administered RNAi therapeutic that silences C5 represents a novel approach to the treatment of complement-mediated diseases. ALN-CC5 utilizes Alnylam's ESC-GalNAc conjugate technology, which enables subcutaneous dosing with increased potency and durability and a wide therapeutic index.