Abstract
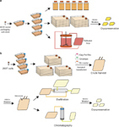
The recent successes of adoptive T-cell immunotherapy for the treatment of hematologic malignancies have highlighted the need for manufacturing processes that are robust and scalable for product commercialization. Here we review some of the more outstanding issues surrounding commercial scale manufacturing of personalized-adoptive T-cell medicinal products. These include closed system operations, improving process robustness and simplifying work flows, reducing labor intensity by implementing process automation, scalability and cost, as well as appropriate testing and tracking of products, all while maintaining strict adherence to Current Good Manufacturing Practices and regulatory guidelines. A decentralized manufacturing model is proposed, where in the future patients’ cells could be processed at the point-of-care in the hospital.
Via Krishan Maggon


 Your new post is loading...
Your new post is loading...








Cancer Gene Therapy (2015) 22, 72–78; doi:10.1038/cgt.2014.78; published online 23 January 2015
Towards a commercial process for the manufacture of genetically modified T cells for therapyOPEN
A D Kaiser1, M Assenmacher1, B Schröder1, M Meyer1, R Orentas2, U Bethke1and B Dropulic2
1Miltenyi Biotec GmbH, Bergisch Gladbach, Germany2Lentigen Technology Inc., Gaithersburg, MD, USACorrespondence: Dr A Kaiser, Miltenyi Biotec GmbH, Friedrich-Ebert-Strasse 68, 51429 Bergisch Gladbach, Germany. E-mail: andrewk@miltenyibiotec.de; B Dropulic, Lentigen Technology Inc., 910 Clopper Road, Gaithersburg, MD, USA. E-mail: boro.dropulic@lentigen.com
Received 22 October 2014; Accepted 5 November 2014
Advance online publication 23 January 2015