Abstract
Chemokines and chemokine receptors orchestrate cell migration and homing in the body. Humans have at least 44 chemokines that are further classified into four subfamilies based on the N-terminal conserved cysteine motifs: CXC, CC, C and CX3C. All the known chemokine receptors are seven transmembrane-type receptors. Humans have 18 chemotactic and 5 atypical non-chemotactic (recycling or scavenging) receptors. CC chemokine receptor 4 (CCR4) is the receptor for two CC chemokine ligands (CCLs)—CCL17 (also called thymus- and activation-regulated chemokine) and CCL22 (macrophage-derived chemokine). Among the various T-cell subsets, CCR4 is predominantly expressed by Th2 cells, cutaneous lymphocyte antigen-positive skin-homing T cells and Treg cells. Thus, CCR4 attracts much attention for its possible clinical applications in diseases involving these T-cell subsets. Furthermore, CCR4 is often highly expressed by mature T-cell neoplasms such as adult T-cell leukemia/lymphoma (ATL) and cutaneous T-cell lymphomas (CTCLs). This article is a brief overview of basic and clinical research on CCR4 and its ligands, which has eventually led to the development of a humanized defucosylated anti-CCR4 antibody ‘Mogamulizumab’ for treatment of relapsed/refractory ATL and CTCLs.
Via Krishan Maggon


 Your new post is loading...
Your new post is loading...



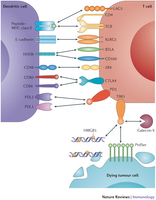






Osamu Yoshie and Kouji MatsushimaCCR4 and its ligands: from bench to bedside
Int. Immunol. (2015) 27 (1): 11-20 doi:10.1093/intimm/dxu079