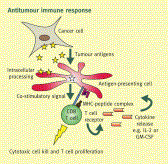
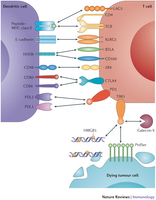
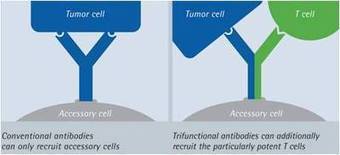
Scientific insights into the human immune system have recently led to unprecedented breakthroughs in immunotherapy. In the twenty-first century, drugs and cell-based therapies developed to bolster humoral and T cell immunity represent an established and growing component of cancer therapeutics. Although natural killer (NK) cells have long been known to have advantages over T cells in terms of their capacity to induce antigen-independent host immune responses against malignancies, their therapeutic potential in the clinic has been largely unexplored. A growing number of scientific discoveries into pathways that both activate and suppress NK cell function, as well as methods to sensitize tumours to NK cell cytotoxicity, have led to the development of numerous pharmacological and genetic methods to enhance NK cell antitumour immunity. These findings, as well as advances in our ability to expand NK cells ex vivoand manipulate their capacity to home to the tumour, have now provided investigators with a variety of new methods and strategies to harness the full potential of NK cell-based cancer immunotherapy in the clinic.
Via Krishan Maggon


 Your new post is loading...
Your new post is loading...











NATURE REVIEWS DRUG DISCOVERY | REVIEW
ARTICLE SERIES: Cancer immunotherapy
Therapeutic approaches to enhance natural killer cell cytotoxicity against cancer: the force awakensRichard W. Childs& Mattias CarlstenAffiliationsCorresponding authorNature Reviews Drug Discovery 14, 487–498 (2015) doi:10.1038/nrd4506Published online 22 May 2015Although I'm not persuing a career in research I would still love to keep up to date the research and discoveries around the scientific community and in particular cancer research and its progress.