The induction of tumor specific humoral immune responses by peptide vaccinations can be achieved by two different approaches: CD4+ T helper peptides for Her-2/neu (with or without the combination of HER-2/neu CD8 epitopes) have been described to induce T cell driven immune responses (DTH; IFN-g production) as well as antibody responses (Disis, 1999 Clin Cancer Res; Disis 2004 J Clin Immunol); however, as these T cell epitopes are HLA restricted such an approach does not allow global usage across all HLA type but requires individual patient selection by HLA typing.
To overcome this problem of HLA restriction, the other approach of peptide vaccination is based on the selection of B cell epitopes located at the extracellular domain (ECD) that give rise to antibodies that specifically bind to regions at the EDC HER-2/neu which inhibit tumor growth by different mechanisms. For the monoclonal antibody Herceptin® several mechanisms of action have been described: antibody-dependent cellular cytotoxicity (ADCC), activation of natural killer cells, internalization and degradation of HER-2/neu, resulting in disruption of receptor dimerization, and suppression of angiogenesis (Nahta R, 2006, Cancer Letter). Therefore it is our intention to induce a polyclonal antibody response with similar anti-tumor properties as Herceptin® by selection of epitopes located within the herceptin binding region. An additional benefit can be expected from inclusion of an epitope which is located within the dimerization loop giving rise to antibodies that inhibit receptor dimerization. Our current multi-epitope vaccine includes such B cell epitopes that meet these described requirements. Indeed, we could prove that the antibodies induced by the vaccine displayed a potent anti-tumor activity via direct tumor cell growth inhibition, and mediation of ADCC and complement dependent cell lysis. (Jasinka J, Int J. Cancer 2003, Wagner S, 2007 Breast Cancer Res Treat).
It has been stated that the effectiveness of humoral anti-tumor responses can be further optimized by IFN-g production by T cells (Lollini P, 2006, Nature Reviews). The important role of IFN-g has been ascribed to induction of antibody isotypes with ADCC mediating properties, activation of effector cells (macrophages and NK cells) and furthermore by inhibiting angiogenesis within tumor lesions. In order to meet these important aspects with our vaccine approach the B cell epitopes were conjugated to a carrier – i.e. virosomes – which has the capacity to induce Th-1 based immune responses (including IFN-g production). In fact, we could demonstrate in our recent phase I trial with the virosomal formulated HER-2/neu multi-peptide vaccine that patients with metastatic breast cancer not only developed anti-HER-2/neu antibodies but also produced significant levels of interferon-g and TNF-a (both classical Th1 cytokines) upon in vitro restimulation with the vaccine carrier (Wiedermann U, 2010, Breast Cancer Res Treat). Additionally, vaccination was associated with a significant decrease of regulatory cells, which might support induction of antitumor-responses by the vaccine.
Via Krishan Maggon


 Your new post is loading...
Your new post is loading...







Taken together, our HER-2/neu multi-peptide immunotherapy offers the following advantages:
1. anti-tumor activity of antibodies induced by B cell epitopes (as described for herceptin)
2. polyclonal responses (superior to treatment with mAbs)
3. HLA independent (advantage over CD8 and CD4 T cell epitopes)
4. induction of T cell responses and cytokine production via effective carrier system
5. Reduction of regulatory cells following vaccination
Image Imugen
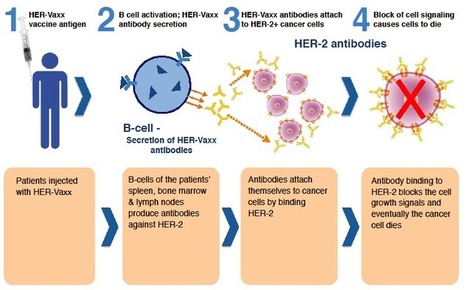
Mechanism of Action
Cancer progression is aided by the ability of tumours to evade recognition by the patient’s own immune system. HER-Vaxx comprises three non-contiguous peptides derived from sequences of HER-2 conjugated to an influenza virosome (adjuvant) that, when injected, activates a patient's own immune system to produce its own polyclonal antibodies, effectively turning the patient's body into a "Herceptin® factory". This antibody response to the HER-2 receptor in turn results in the death of the tumour cells. The following diagram illustrates how HER-Vaxx is intended to work: