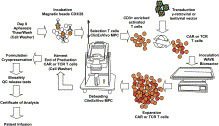
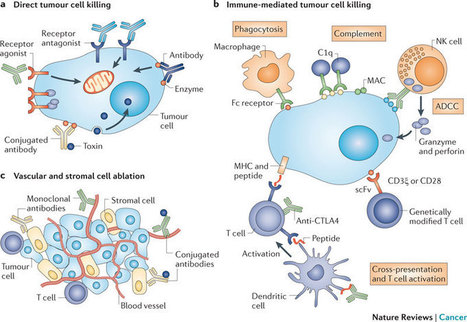
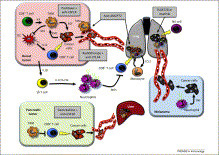
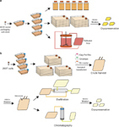
The promising clinical results obtained with engineered T cells, including chimeric antigen receptor (CAR) therapy, call for further advancements to facilitate and broaden their applicability. One potentially beneficial innovation is to exploit new T cell sources that reduce the need for autologous cell manufacturing and enable cell transfer across histocompatibility barriers. Here we review emerging T cell engineering approaches that utilize alternative T cell sources, which include virus-specific or T cell receptor-less allogeneic T cells, expanded lymphoid progenitors, and induced pluripotent stem cell (iPSC)-derived T lymphocytes. The latter offer the prospect for true off-the-shelf, genetically enhanced, histocompatible cell therapy products.
Via Krishan Maggon


 Your new post is loading...
Your new post is loading...








Volume 16, Issue 4, 2 April 2015, Pages 357–366
3601||
Perspective New Cell Sources for T Cell Engineering and Adoptive ImmunotherapyMaria Themeli1, Isabelle Rivière1, Michel Sadelain1, , doi:10.1016/j.stem.2015.03.011