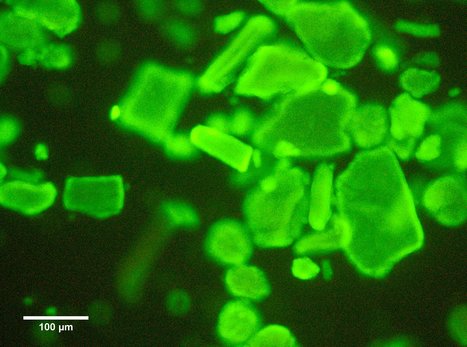
Type A Carbohydrate-Binding Modules (CBMs) are a critical component of cellulose-degrading microbial enzymes, but their mechanism of action remains poorly understood. In a paper just published in the Journal of Biological Chemistry, Miguel Prazeres and Pedro Pereira from BERG-iBB, working with researchers from the Faculty of Veterinary Medicine, Nova University and NZYTech describe the production and characterization of a library of recombinant CBMs representative of the known diversity of type A modules. The binding properties of 40 CBMs, in fusion with an N-terminal green fluorescence protein (GFP) domain, revealed that type A CBMs possess the ability to recognize different crystalline forms of cellulose and chitin over a wide range of temperatures, pHs and ionic strengths. A Spirochaeta thermophila CBM64, in particular, displayed plasticity in its capacity to bind both crystalline and soluble carbohydrates under a wide range of extreme conditions. The structure of S. thermophila StCBM64C was further solved, revealing an untwisted, flat, carbohydrate-binding interface comprising the side chains of four tryptophan residues in a coplanar linear arrangement. Click on title to learn more.
No comment yet.
Sign up to comment



 Your new post is loading...
Your new post is loading...