 Your new post is loading...
 Your new post is loading...
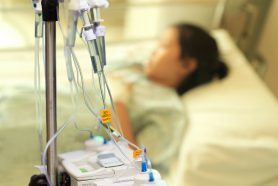
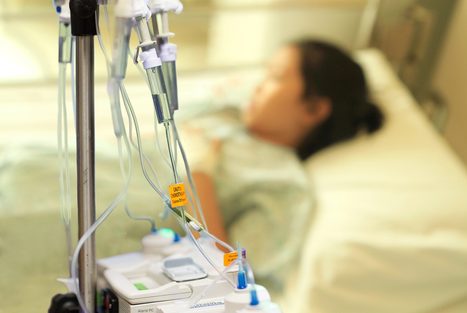
The FDA has mandated that the boxed warning for all approved CAR T-cell therapies be updated to include the serious risk of T-cell malignancies.
In a remarkable study, researchers have developed a novel CRISPR-Cas9 delivery system utilising cryo-shocked lung tumour cells to target non-small cell lung cancer (NSCLC), particularly those with KRAS mutations. This innovative method, leveraging the concept of synthetic lethality, shows promise in improving the targeting efficiency o
RS Genomics Limited, which was formed to provide broad access to the foundational CRISPR/Cas9 intellectual property co-owned by Dr. Emmanuelle Charpentier, today announced its patent CN201380038920.6 was upheld by the CNIPA in response to an invalidation challenge.
Researchers at the University of Zurich and the University Hospital Zurich have discovered that a specific mutation in the cancer cells of an aggressive type of blood cancer can prevent novel immunotherapies such as CAR T-cell therapy from working.
A new type of immunotherapy that targets non-cancer cells could help prevent the growth and spread of breast cancer tumors, according to new research funded by Breast Cancer Now.
Jan Joseph Melenhorst, PhD, emphasizes how research investigating chronic lymphocytic leukemia biology may result in more targeted CAR T-cell products.
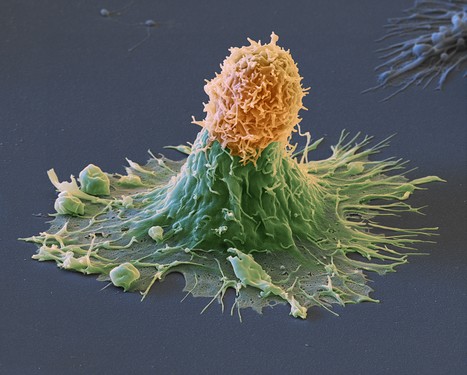
Scientists at the UCLA Health Jonsson Comprehensive Cancer Center have built and demonstrated the potential efficacy of a new chimeric antigen receptor (CAR) T-cell-based immunotherapy specifically designed to treat patients with cutaneous and rare subtypes of melanoma.
Intellia Therapeutics shares interim clinical results from the Phase 1 portion of the ongoing Phase 1/2 trial of NTLA-2002 in hereditary angioedema (HAE). NTLA-2002 is an investigational in vivo CRISPR-based gene editing therapy in development as a single-dose treatment for HAE, and the results were were published online in the Ne
The development of any type of second cancer following CAR T cell therapy is a rare occurrence, as found in an analysis of more than 400 patients treated at Penn Medicine, researchers from the Perelman School of Medicine at the University of Pennsylvania reported today in Nature Medicine.
BRL-201 had a manageable safety profile and elicited early indications of efficacy in patients with relapsed/refractory non-Hodgkin lymphoma.
The FDA has approved label updates for zanubrutinib to include data from the ALPINE trial in relapsed/refractory chronic lymphocytic leukemia, and axicabtagene ciloleucel to include findings from the primary overall survival analysis of the ZUMA-7 trial in relapsed/refractory large B-cell lymphoma.
Patent JP6692856 upheld for second time in response to invalidation challenge.
The Malaghan Institute of Medical Research in collaboration with Wellington Zhaotai Therapies Limited today announced results of its phase 1 dose escalation trial of a new third-generation anti-CD19 chimeric antigen receptor (CAR) T-cell therapy to be presented at the American Society of Hematology (ASH) Annual Meeting in San Diego on 11 December, 3pm.
|
The FDA approved ciltacabtagene autoleucel for earlier use in certain adults with relapsed or refractory multiple myeloma. The expanded indication permits treatment with the chimeric antigen receptor T cells after first relapse and applies to adults who are refractory to lenalidomide and have received at least one previous line of therapy, including a proteasome inhibitor and an immunomodulatory
Researchers have unveiled a novel method utilising in vivo cleavable donor plasmids for CRISPR-Cas9 and CRISPR-Cas12a, significantly enhancing gene editing efficiency in adult mouse livers. This method employs hydrodynamic delivery of targeting plasmids, showcasing a substantial improvement over traditional techniques.
A study aiming to develop a low-cost, rapid detection technique for the widescale detection and screening of oral microorganisms suitable for point-of-care settings was presented at the 102nd General Session of the IADR, which was held in conjunction with the 53rd Annual Meeting of the American Association for Dental, Oral, and Craniofacial Research and the 48th Annual Meeting of the Canadian Association for Dental Research, on March 13-16, 2024, in New Orleans, LA, USA.
Breakthrough in Brain Cancer Treatment: Dual-Target CAR T Cell Therapy Shows Promise in Early Clinical Trial.
A specific gene may play a key role in new treatments that prevent muscle in the body from breaking down in serious muscle diseases, muscular dystrophies.
AI tools that can come up with protein structures at the push of a button should be used safely and ethically, say researchers in the field.
Belgian researchers from VIB-KU Leuven Center for Microbiology and VIB-UGent Center for Plant Systems Biology have developed a new toolbox of 16 different short DNA sequences that allow triggering controlled and specific recombination events in any genome.
Liso-cel sBLAs for indications in relapsed/refractory FL and MCL after exposure to a BTK inhibitor have received priority review from the FDA.
Researchers at Universidade de São Paulo in Brazil review progress in recent years involving the use of gene editing to cure HIV and prevent associated neurocognitive disorders.
The cancer research landscape is transforming by introducing novel mouse models utilising prime- and base-editing techniques. Yesterday, a News & Views in Nature Biotechnology summarised the findings of two recent studies that carefully examine the role of specific genetic variants in cancer.
Humans can benefit significantly from symbiotic relationships with probiotics-;live bacteria and microorganisms that influence the gut microbiota.
|






 Your new post is loading...
Your new post is loading...










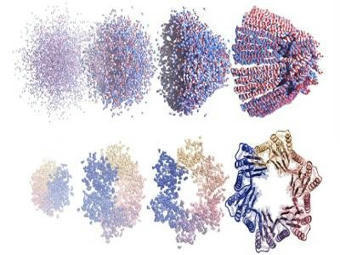







CROPSR is the first open source software tool for genome-wide design and evaluation of guide RNA (gRNA) sequences for CRISPR experiments, created by scientists at CABBI, a Department of Energy-funded Bioenergy Research Center (BRC). The genome-wide approach significantly shortens the time needed to design a CRISPR experiment, reducing the challenge of working with crops and speeding up the design, evaluation and validation of gRNA sequences, according to the study published in BMC Bioinformatics. To better meet the needs of crop geneticists, the team built software that lifts restrictions imposed by other packages on the design and evaluation of gRNA sequences, the guides used to locate targeted genetic material. Team members also developed a new machine learning model that would not avoid guides for repetitive genomic regions often found in plants, a problem with existing tools. The CROPSR scoring model provided much more accurate predictions, even in non-crop genomes. In the future, he hopes researchers will record their failures as well as their successes to help generate the data needed to train a non-specific model.