The FDA has entered into the federal register a new draft guidance pertaining to "software as a medical device" (SaMD). The guidance is presented as representing the FDA's current thinking on establishing clinical evaluation guidelines for SaMD, but is written by an international organization of device regulators, the International Medical Device Regulators Forum, of which FDA is a member.
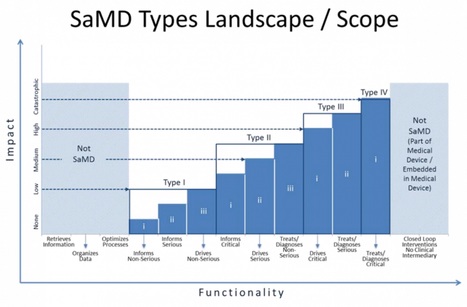
The guidance seeks to articulate what's new and different about SaMD (a category which would include mobile medical apps) and provide a stratified guidance on how to regulate different kinds of software and what kind of evidence is needed for each regulatory category. The guidance stratifies devices on two axes: whether the device informs care, drives care, or treats/diagnoses and whether the condition in question is non-serious, serious, or critical. So software that treats or diagnoses a critical condition is in the highest risk category, while software that informs care about a non-serious condition is in the lowest.
The guidelines also call out and address the fact that software development tends to move faster than traditional medical device development and can more easily be influenced by postmarket data.
"SaMD ... is unique in that it operates in a complex highly connected-interactive socio-technical environment in which frequent changes and modifications can be implemented more quickly and efficiently," the guidance says. "Development of SaMD is also heavily influenced by new entrants unfamiliar with medical device regulations and terminology developing a broad spectrum of applications."
Via Pharma Guy



 Your new post is loading...
Your new post is loading...







Related article: “Is SaaD - Software as a Drug - the Next Big Thing in mHealth?”; http://sco.lt/7SITsv
Via @pharmaguy The New #FDA draft guidance on software as a Medical Device (SaMD)