Your new post is loading...
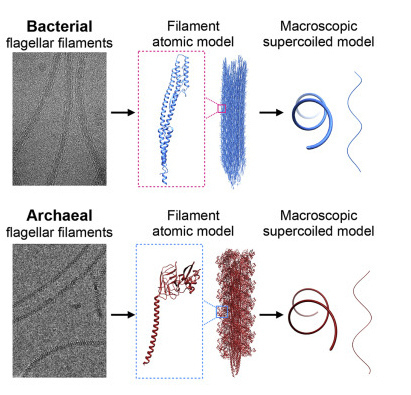
University of Virginia School of Medicine researchers and their collaborators have solved a decades-old mystery about how E. coli and other bacteria are able to move. Bacteria push themselves forward by coiling long, threadlike appendages into corkscrew shapes that act as makeshift propellers. But how exactly they do this has baffled scientists, because the "propellers" are made of a single protein. An international team led by UVA's Edward H. Egelman, PhD, a leader in the field of high-tech cryo-electron microscopy (cryo-EM), has cracked the case. The researchers used cryo-EM and advanced computer modeling to reveal what no traditional light microscope could see: the strange structure of these propellers at the level of individual atoms. "While models have existed for 50 years for how these filaments might form such regular coiled shapes, we have now determined the structure of these filaments in atomic detail," said Egelman, of UVA's Department of Biochemistry and Molecular Genetics. "We can show that these models were wrong, and our new understanding will help pave the way for technologies that could be based upon such miniature propellers." Blueprints for Bacteria's 'Supercoils' Different bacteria have one or many appendages known as a flagellum, or, in the plural, flagella. A flagellum is made of thousands of subunits, but all these subunits are exactly the same. You might think that such a tail would be straight, or at best a bit flexible, but that would leave the bacteria unable to move. That's because such shapes can't generate thrust. It takes a rotating, corkscrew-like propeller to push a bacterium forward. Scientists call the formation of this shape "supercoiling," and now, after more than 50 years, they understand how bacteria do it. Using cryo-EM, Egelman and his team found that the protein that makes up the flagellum can exist in 11 different states. It is the precise mixture of these states that causes the corkscrew shape to form. It has been known that the propeller in bacteria is quite different than similar propellers used by hearty one-celled organisms called archaea. Archaea are found in some of the most extreme environments on Earth, such as in nearly boiling pools of acid, the very bottom of the ocean and in petroleum deposits deep in the ground. Egelman and colleagues used cryo-EM to examine the flagella of one form of archaea, Saccharolobus islandicus, and found that the protein forming its flagellum exists in 10 different states. While the details were quite different than what the researchers saw in bacteria, the result was the same, with the filaments forming regular corkscrews. They conclude that this is an example of "convergent evolution" -- when nature arrives at similar solutions via very different means. This shows that even though bacteria and archaea's propellers are similar in form and function, the organisms evolved those traits independently. "As with birds, bats and bees, which have all independently evolved wings for flying, the evolution of bacteria and archaea has converged on a similar solution for swimming in both," said Egelman, whose prior imaging work saw him inducted into the National Academy of Sciences, one of the highest honors a scientist can receive. "Since these biological structures emerged on Earth billions of years ago, the 50 years that it has taken to understand them may not seem that long."
In a major scientific "quantum" leap, University of Queensland researchers have created a quantum microscope that can reveal biological structures that would otherwise be impossible to see. This paves the way for applications in biotechnology, and could extend far beyond this into areas ranging from navigation to medical imaging. The microscope is powered by the science of quantum entanglement, an effect Einstein described as "spooky interactions at a distance." Professor Warwick Bowen, from UQ's Quantum Optics Lab and the ARC Centre of Excellence for Engineered Quantum Systems (EQUS), said it was the first entanglement-based sensor with performance beyond the best possible existing technology. "This breakthrough will spark all sorts of new technologies -- from better navigation systems to better MRI machines, you name it," Professor Bowen said. "Entanglement is thought to lie at the heart of a quantum revolution. We've finally demonstrated that sensors that use it can supersede existing, non-quantum technology. This is exciting -- it's the first proof of the paradigm-changing potential of entanglement for sensing." Australia's Quantum Technologies Roadmap sees quantum sensors spurring a new wave of technological innovation in healthcare, engineering, transport and resources. A major success of the team's quantum microscope was its ability to catapult over a 'hard barrier' in traditional light-based microscopy. "The best light microscopes use bright lasers that are billions of times brighter than the sun," Professor Bowen said. "Fragile biological systems like a human cell can only survive a short time in them and this is a major roadblock. The quantum entanglement in our microscope provides 35 per cent improved clarity without destroying the cell, allowing us to see minute biological structures that would otherwise be invisible. The benefits are obvious -- from a better understanding of living systems, to improved diagnostic technologies." Professor Bowen said there were potentially boundless opportunities for quantum entanglement in technology. "Entanglement is set to revolutionize computing, communication and sensing," he said. "Absolutely secure communication was demonstrated some decades ago as the first demonstration of absolute quantum advantage over conventional technologies. Computing faster than any possible conventional computer was demonstrated by Google two years ago, as the first demonstration of absolute advantage in computing. The last piece of the puzzle was sensing, and we've now closed that gap."
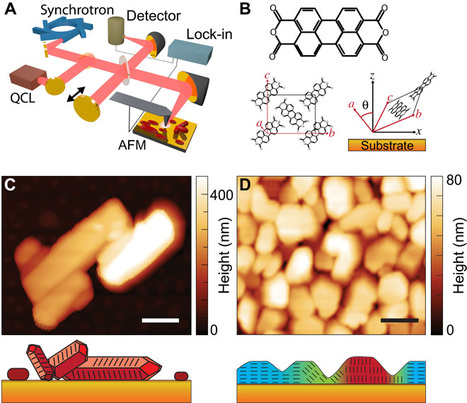
Unprecedented details of enamel structure may point to new ways to prevent or halt cavities. Scientists used a combination of advanced microscopy and chemical detection techniques to uncover the structural makeup of human tooth enamel at unprecedented atomic resolution, revealing lattice patterns and unexpected irregularities. The findings could lead to a better understanding of how tooth decay develops and might be prevented. The research was supported in part by the National Institute of Dental and Craniofacial Research (NIDCR) at the National Institutes of Health. The findings appear in Nature. “This work provides much more detailed information about the atomic makeup of enamel than we previously knew,” said Jason Wan, Ph.D., a program officer at NIDCR. “These findings can broaden our thinking and approach to strengthening teeth against mechanical forces, as well as repairing damage due to erosion and decay.” Your teeth are remarkably resilient, despite enduring the stress and strain of biting, chewing, and eating for a lifetime. Enamel — the hardest substance in the human body — is largely responsible for this endurance. Its high mineral content gives it strength. Enamel forms the outer covering of teeth and helps prevent tooth decay, or caries. Tooth decay is one of the most common chronic diseases, affecting up to 90% of children and the vast majority of adults worldwide, according to the World Health Organization. Left untreated, tooth decay can lead to painful abscesses, bone infection, and bone loss. Tooth decay starts when excess acid in the mouth erodes the enamel covering. Scientists have long sought a more complete picture of enamel’s chemical and mechanical properties at the atomic level to better understand—and potentially prevent or reverse—enamel loss. To survey enamel at the tiniest scales, researchers use microscopy methods such as scanning transmission electron microscopy (STEM), which directs a beam of electrons through a material to map its atomic makeup.
University of British Columbia researchers have developed a specialized microscope that has the potential ability to both diagnose diseases that include skin cancer and perform incredibly precise surgery—all without cutting skin. The researchers describe the technology in a study published today in Science Advances. “Our technology allows us to scan tissue quickly, and when we see a suspicious or abnormal cell structure, we can perform ultra-precise surgery and selectively treat the unwanted or diseased structure within the tissue—without cutting into the skin,” said Yimei Huang, co-lead author of the study and a former postdoctoral fellow at the department of dermatology and skin science at UBC and BC Cancer. Huang co-led the study with Zhenguo Wu, a UBC PhD student. The device is a specialized type of multiphoton excitation microscope that allows imaging of living tissue up to about one millimeter in depth using an ultrafast infrared laser beam. What sets the researchers’ microscope apart from previous technology is that it’s capable of not only digitally scanning living tissue, but also treating the tissue by intensifying the heat produced by the laser. When applied to treating diseases of the skin, the microscope allows medical professionals to pinpoint the exact location of the abnormality, diagnose it and treat it instantly. It could be used to treat any structure of the body that is reached by light and that requires extremely precise treatment, including nerves or blood vessels in the skin, eye, brain or other vital structures. “We can alter the pathway of blood vessels without impacting any of the surrounding vessels or tissues,” said study co-author Harvey Lui, professor at the department of dermatology and skin science at UBC and the Vancouver Coastal Health Research Institute, and a dermatologist at BC Cancer. “For diagnosing and scanning diseases like skin cancer, this could be revolutionary.”
New research published in Nature Methods will dramatically improve how scientists "see inside" molecular structures in solution, allowing for much more precise ways to image data in various fields, from astronomy to drug discovery. The new method will allow for the visualization of many more biological molecules, providing critical information about what is inside molecules to scientists who currently can only access their outer shape or envelope. Such information could be a major boost to studies of viruses, for example. "With existing techniques, you can only see the outline of the virus," said author Thomas D. Grant, PhD, research assistant professor in the Department of Structural Biology in the Jacobs School of Medicine and Biomedical Sciences at the University at Buffalo and the Department of Materials, Design and Innovation in the UB School of Engineering and Applied Sciences and Hauptman-Woodward Medical Research Institute. "This new method allows us to see inside the virus molecule to understand how the genetic information is arranged, potentially giving new insight into how the virus injects this genetic information into its host." Grant is the sole author of the paper, a rarity among papers published in this journal. He is a scientist with BioXFEL (Biology with X-ray Free Electron Lasers), a National Science Foundation Science and Technology Center composed of eight U.S. research universities that is headquartered at UB. Its mission is to address fundamental questions in biology at the molecular level using cutting-edge techniques, including X-ray laser science. Grant's method has solved the phase problem for a particular molecular determination technique called solution scattering. The phase problem is where critical information about the phase of a molecule is lost during the experimental process of making a physical measurement. He explained that most molecular structures today are solved using X-ray crystallography, where the structures scatter intense X-rays in patterns consisting of hundreds of thousands of unique pieces of information, which are used to ultimately reveal the structure at high-resolution. "The problem is that more than 75 percent of molecular structures do not readily form the ordered crystals that diffract well," explained Grant. "That means many molecules are difficult to visualize in three dimensions." In addition, he said, biological molecules can exhibit dynamic motions that have an impact on how they function but those motions are missing when structures crystallize, resulting in the loss of important biological information. One way around this obstacle is to use a technique called solution scattering in which X-rays scatter off of molecules floating in solution instead of arranged in a crystal. "Solution scattering allows the molecules to move dynamically in their natural states, enabling the visualization of large-scale conformational dynamics important for biological function," said Grant. "However, as the molecules tumble in solution, they scatter the X-rays in many different orientations, losing most of the information, typically yielding only 10 to 20 unique pieces of data." Until now, such little information only yielded low-resolution outlines of the particle shape.
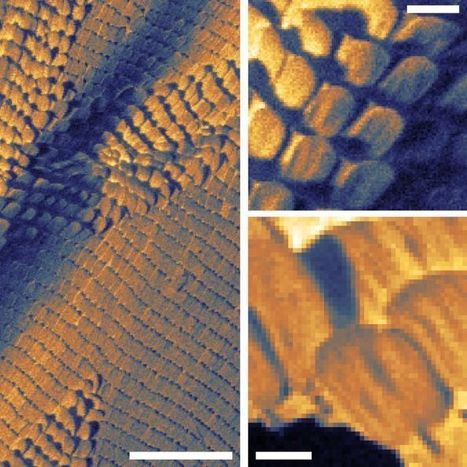
Atomic force microscopy (AFM) is an extremely sensitive technique that allows us to image materials and/or characterize their physical properties on the atomic scale by sensing the force above material surfaces using a precisely controlled tip. However, conventional AFM only provides the surface normal component of the force (the Z direction) and ignores the components parallel to the surface (the X and Y directions). To fully characterize materials used in nanoscale devices, it is necessary to obtain information about parameters with directionality, such as electronic, magnetic, and elastic properties, in more than just the Z direction. That is, it is desirable to measure these parameters in the X and Y directions parallel to the surface of a material as well. Measuring the distribution of such material parameters on the atomic scale will increase our understanding of chemical composition and reactions, surface morphology, molecular manipulation, and nanomachine operation. A research group at Osaka University has recently developed an AFM-based approach called "bimodal AFM" to obtain information about material surfaces in the X, Y, and Z directions (that is, in three dimensions) on the subatomic scale. The researchers measured the total force between an AFM tip and material surface in the X, Y, and Z directions using a germanium (Ge) surface as a substrate. Their collaborative partner, the Institute of Physics of the Slovak Academy of Sciences, contributed computer simulations of the tip-surface interactions. The bimodal AFM approach was recently reported in Nature Physics. "A clean Ge(001) surface has alternately aligned anisotropic dimers, which are rotated by 90° across the step, meaning they show a two-domain structure," explains first author Yoshitaka Naitoh. "We probed the force fields from each domain in the vertical direction by oscillating the AFM tip at the flexural resonance frequency and in the parallel direction by oscillating it at the torsional one." The team first expressed the force components as vectors, providing the vector distribution above the surface at the subatomic scale. The computer simulation supported the experimental results and shed light on the nature of chemical tip termination and morphology and, in particular, helped to clarify the outstanding questions regarding the tip-surface distances in the experiment. "We measured the magnitude and direction of the force between the AFM tip and Ge surface on a subatomic scale in three dimensions," says Naitoh. "Such measurements will aid understanding of the structure and chemical reactions of functionalized surfaces."
A new, ;ow-cost, ten-times-higher-resolution spectroscopy technique could allow for detection of microscopic amounts of chemicals for applications in security, law enforcement, and research. MIT researchers have developed a radical design for a low-cost, miniaturized microscope that can chemically identify individual micrometer-sized particles. It could one day be used in airports or other high-security venues as a highly sensitive and low-cost way to rapidly screen people for microscopic amounts of potentially dangerous materials. It could also be used for scientific analysis of very small samples or for measuring the optical properties of materials. In an open-access paper in the journal Optics Letters, from The Optical Society (OSA), the researchers demonstrated their new “photothermal modulation of Mie scattering” (PMMS) microscope by measuring infrared spectra of individual 3-micrometer spheres made of silica or acrylic. The new technique uses a simple optical setup consisting of compact components that will allow the instrument to be miniaturized into a portable device about the size of a shoebox. The new microscope’s use of visible wavelengths for imaging gives it a spatial resolution of around 1 micrometer, compared to the roughly 10-micrometer resolution of traditional infrared spectroscopy methods. This increased resolution allows the new technique to distinguish and identify individual particles that are extremely small and close together.* “If there are two very different particles in the field of view, we’re able to identify each of them,” said Stolyarov. “This would never be possible with a conventional infrared technique because the image would be indistinguishable.” “The most important advantage of our new technique is its highly sensitive, yet remarkably simple design,” said Ryan Sullenberger, associate staff at MIT Lincoln Labs and first author of the paper. “It provides new opportunities for nondestructive chemical analysis while paving the way towards ultra-sensitive and more compact instrumentation.”
Molecular solids and polymers can form low-symmetry crystal structures that exhibit anisotropic electron and ion mobility in engineered devices or biological systems. The distribution of molecular orientation and disorder then controls the macroscopic material response, yet it is difficult to image with conventional techniques on the nanoscale. Scientists now have demonstrated a new form of optical nanocrystallography that combines scattering-type scanning near-field optical microscopy with both optical antenna and tip-selective infrared vibrational spectroscopy. From the symmetry-selective probing of molecular bond orientation with nanometer spatial resolution, they determined crystalline phases and orientation in aggregates and films of the organic electronic material perylenetetracarboxylic dianhydride. Mapping disorder within and between individual nanoscale domains, the correlative hybrid imaging of nanoscale heterogeneity provides insight into defect formation and propagation during growth in functional molecular solids.
Scientists at The University of Texas at Austin have demonstrated a method for making three-dimensional images of structures in biological material under natural conditions at a much higher resolution than other existing methods. The method may help shed light on how cells communicate with one another and provide important insights for engineers working to develop artificial organs such as skin or heart tissue. The research is described today in the journal Nature Communications. The scientists, led by physicist Ernst-Ludwig Florin, used their method, called thermal noise imaging, to capture nanometer-scale images of networks of collagen fibrils, which form part of the connective tissue found in the skin of animals. A nanometer is a billionth of a meter or about one-hundred-thousandth of the width of a human hair. Examining collagen fibrils at this scale allowed the scientists to measure for the first time key properties that affect skin's elasticity, something that could lead to improved designs for artificial skin or tissues.
Researchers have developed a new enhanced DNA imaging technique that can probe the structure of individual DNA strands at the nanoscale. Since DNA is at the root of many disease processes, the technique could help scientists gain important insights into what goes wrong when DNA becomes damaged or when other cellular processes affect gene expression. The new imaging method builds on a technique called single-molecule microscopy by adding information about the orientation and movement of fluorescent dyes attached to the DNA strand. W. E. Moerner, Stanford University, USA, is the founder of single-molecule spectroscopy, a breakthrough method from 1989 that allowed scientists to visualize single molecules with optical microscopy for the first time. Of the 2014 Nobel Laureates for optical microscopy beyond the diffraction limit (Moerner, Hell & Betzig), Moerner and Betzig used single molecules to image a dense array of molecules at different times. In The Optical Society's journal for high impact research, Optica, the research team led by Moerner describes their new technique and demonstrates it by obtaining super-resolution images and orientation measurements for thousands of single fluorescent dye molecules attached to DNA strands. "You can think of these new measurements as providing little double-headed arrows that show the orientation of the molecules attached along the DNA strand," said Moerner. "This orientation information reports on the local structure of the DNA bases because they constrain the molecule. If we didn't have this orientation information the image would just be a spot." A strand of DNA is a very long, but narrow string, just a few nanometers across. Single-molecule microscopy, together with fluorescent dyes that attach to DNA, can be used to better visualize this tiny string. Until now, it was difficult to understand how those dyes were oriented and impossible to know if the fluorescent dye was attached to the DNA in a rigid or somewhat loose way. Adam S. Backer, first author of the paper, developed a fairly simple way to obtain orientation and rotational dynamics from thousands of single molecules in parallel. "Our new imaging technique examines how each individual dye molecule labeling the DNA is aligned relative to the much larger structure of DNA," said Backer. "We are also measuring how wobbly each of these molecules is, which can tell us whether this molecule is stuck in one particular alignment or whether it flops around over the course of our measurement sequence."
Via Mariaschnee
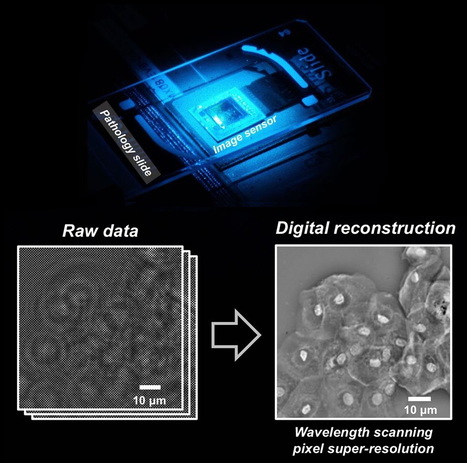
Researchers from the California NanoSystems Institute at UCLA have created a new technique using lens-free holograms that greatly enhances digital microscopy images, which are sometimes blurry and pixelated.
The new technique, called “wavelength scanning pixel super-resolution,” uses a device that captures a stack of digital images of the same specimen, each with a slightly different wavelength of light. Then, researchers apply a newly devised algorithm that divides the pixels in each captured image into a number of smaller pixels, resulting in a much higher-resolution digital image of the specimen.
The research team was led by Aydogan Ozcan, Chancellor’s Professor of Electrical Engineering and Bioengineering at the UCLA Henry Samueli School of Engineering and Applied Science. The study appears in an open-access paper in the journal Light: Science and Applications, published by the Nature Publishing Group.
“These results mean we can see and inspect large samples with finer details at the sub-micron [nanoscale] level,” Ozcan said. “We have applied this method to lens-based conventional microscopes, as well as our lensless on-chip microscopy systems that create microscopic images using holograms, and it works across all these platforms.”
The benefits of this new method are wide-ranging, but especially significant in pathology, where rapid microscopic imaging of large numbers of tissue or blood cells is key to diagnosing diseases such as cancer. The specimens used in the study were blood samples, used to screen for various diseases, and Papanicolaou tests, which are used to screen for cervical cancer.
Ozcan said that wavelength scanning super-resolution works on both colorless and dye-stained samples. The entire apparatus fits on a desktop, so its size and convenience could be of great benefit to doctors and scientists using microscopes in resource-limited settings such as clinics in developing countries.
Today’s digital photos are far more vivid than just a few years ago, thanks to a steady stream of advances in optics, detectors, and software. Similar advances have also improved the ability of machines called cryo-electron microscopes (cryo-EMs) to see the Lilliputian world of atoms and molecules. Now, researchers report that they’ve created the highest ever resolution cryo-EM image, revealing a druglike molecule bound to its protein target at near atomic resolution. The resolution is so sharp that it rivals images produced by x-ray crystallography, long the gold standard for mapping the atomic contours of proteins. This newfound success is likely to dramatically help drugmakers design novel medicines for a wide variety of conditions.
“This represents a new era in imaging of proteins in humans with immense implications for drug design,” says Francis Collins, who heads the U.S. National Institutes of Health in Bethesda, Maryland. Collins may be partial. He’s the boss of the team of researchers from the National Cancer Institute (NCI) and the National Heart, Lung, and Blood Institute that carried out the work. Still, others agree that the new work represents an important milestone. “It’s a major advance in the technology,” says Wah Chiu, a cryo-EM structural biologist at Baylor College of Medicine in Houston, Texas. “It shows [cryo-EM] technology is here.”
Cryo-EM has long seemed behind the times—an old hand tool compared with the modern power tools of structural biology. The two main power tools, x-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy, enable researchers to pin down the position of protein features to less than 0.2 nanometers, good enough to see individual atoms. By contrast, cryo-EM has long been limited to a resolution of 0.5 nm or more.
Cryo-EM has been around for decades. But until recently its resolution hasn’t even been close to crystallography and NMR. “We used to be called the field of blob-ology,” says Sriram Subramaniam, a cryo-EM structural biologist at NCI, who led the current project. But steady improvements to the electron beam generators, detectors, and imaging analysis software have slowly helped cryo-EM inch closer to the powerhouse techniques. Earlier this year, for example, two groups of researchers broke the 0.3-nm-resolution benchmark, enough to get a decent view of the side arms of two proteins’ individual amino acids. Still, plenty of detail in the images remained fuzzy.
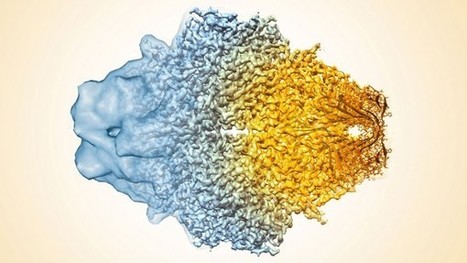
For their current study, Subramaniam and his colleagues sought to refine their images of β-galactosidase, a protein they imaged last year at a resolution of 0.33 nm. The protein serves as a good test case, Subramaniam says, because researchers can compare their images to existing x-ray structures to check their accuracy. Subramaniam adds that the current advance was more a product of painstaking refinements to a variety of techniques—including protein purification procedures that ensure each protein copy is identical and software improvements that allow researchers to better align their images. Subramaniam and his colleagues used some 40,000 separate images to piece together the final shape of their molecule. They report online today in Science that these refinements allowed them to produce a cryo-EM image of β-galactosidase at a resolution of 0.22 nm, not quite sharp enough to see individual atoms, but clear enough to see water molecules that bind to the protein in spots critical to the function of the molecule.
The above composite image of the protein β-galactosidase shows the progression of cryo-EM’s ability to resolve a protein’s features from mere blobs (left) a few years ago to the ultrafine 0.22-nanometer resolution today (right).
Researchers at the University of Tokyo have succeeded in developing a new microscope capable of observing the magnetic sensitivity of photochemical reactions believed to be responsible for the ability of some animals to navigate in Earth's magnetic field, on a scale small enough to follow these reactions taking place inside sub-cellular structures.
Several species of insects, fish, birds and mammals are believed to be able to detect magnetic fields -- an ability known as magnetoreception. For example, birds are able to sense Earth's magnetic field and use it to help navigate when migrating. Recent research suggests that a group of proteins called cryptochromes and particularly the molecule flavin adenine dinucleotide (FAD) that forms part of the cryptochrome, are implicated in magnetoreception. When cryptochromes absorb blue light, they can form what are known as radical pairs. The magnetic field around the cryptochromes determines the spins of these radical pairs, altering their reactivity. However, to date there has been no way to measure the effect of magnetic fields on radical pairs in living cells.
The research group of Associate Professor Jonathan Woodward at the Graduate School of Arts and Sciences are specialists in radical pair chemistry and investigating the magnetic sensitivity of biological systems. In this latest research, PhD student Lewis Antill made measurements using a special microscope to detect radical pairs formed from FAD, and the influence of very weak magnetic fields on their reactivity, in volumes less than 4 millionths of a billionth of a liter (4 femtoliters). This was possible using a technique the group developed called TOAD (transient optical absorption detection) imaging, employing a microscope built by postdoctoral research associate Dr. Joshua Beardmore based on a design by Beardmore and Woodward.
"In the future, using another mode of the new microscope called MIM (magnetic intensity modulation), also introduced in this work, it may be possible to directly image only the magnetically sensitive regions of living cells," says Woodward. "The new imaging microscope developed in this research will enable the study of the magnetic sensitivity of photochemical reactions in a variety of important biological and other contexts, and hopefully help to unlock the secrets of animals' miraculous magnetic sense."
|
If you open a biology textbook and run through the images depicting how DNA is organized in the cell's nucleus, chances are you'll start feeling hungry; the chains of DNA would seem like a bowl of ramen: long strings floating in liquid. However, according to two new studies—one experimental and the other theoretical—that are the outcome of the collaboration between the groups of Prof. Talila Volk of the Molecular Genetics Department and Prof. Sam Safran of the Chemical and Biological Physics Department at the Weizmann Institute of Science, this image should be reconsidered. Clarifying it is essential since DNA's spatial arrangement in the nucleus can affect the expression of genes contained within the DNA molecule, and hence the proteins found in the cell. This story began when Volk was studying how mechanical forces influence cell nuclei in the muscle and found evidence that muscle contractions had an immediate effect on gene expression patterns. "We couldn't explore this further because existing methods relied on imaging of chemically preserved cells, so they failed to capture what happens in the cell nuclei of an actual working muscle," she says. To address this issue, Dr. Dana Lorber, a research associate in Volk's group, led the design of a device that makes it possible to study muscle nuclei in live fruit fly larvae. The device holds the tiny, translucent larva within a groove that allows it to contract and relax its muscles but keeps its movement constrained so that it can be scanned by a fluorescence microscope. Using the device, the researchers obtained images of the internal, linearly-organized complexes of DNA and its proteins (known as chromatin), surrounded by the membrane of the muscle nuclei. Expecting a bowl full of ramen, Lorber and Dr. Daria Amiad-Pavlov, a postdoctoral fellow in Volk's group, were in for a surprise. Rather than filling up the entire volume of the nucleus, the "noodles," or long chromatin molecules, were organized as a relatively thin layer, attached to its inner walls. Similar to the outcome of the interaction between oil and water, what is known as "phase separation," the chromatin separated itself from the bulk of the liquid inside of the nucleus and found its place at its outskirts, while most of the fluid medium remained at the center. The researchers realized that they were on their way to addressing a fundamental biological question, that is—how is chromatin, and hence DNA, organized in the nucleus in a living organism. "But the findings were so unexpected, we had to make sure no error had crept in and that this organization was universal," Lorber says.
A fast, low-cost technique to see and count viruses or proteins from a sample in real time, without any chemicals or dyes, could underpin a new class of devices for rapid diagnostics and viral load monitoring, including HIV and the virus that causes COVID-19. Researchers at the University of Illinois Urbana-Champaign described the technique, called Photonic Resonator Interferometric Scattering Microscopy, or PRISM, in the journal Nature Communications. “We have developed a new form of microscopy that amplifies the interaction between light and biological materials. We can use it for very rapid and sensitive forms of diagnostic testing, and also as a very powerful tool for understanding biological processes at the scale of individual items, like counting individual proteins or recording individual protein interactions,” said study leader Brian Cunningham, the Intel Alumni Endowed Chair of electrical and computer engineering at Illinois. In optical microscopes, light bounces off any molecules or viruses it encounters on a slide, creating a signal. Instead of a regular glass slide, the PRISM technique uses a photonic crystal — a nanostructured glass surface that brilliantly reflects only one specific wavelength of light. Cunningham’s group designed and fabricated a photonic crystal that reflects red light, so that the light from a red laser would be amplified. “The molecules we are looking at – in this study, viruses and small proteins – are extremely small. They cannot scatter enough light to create a signal that can be detected by a conventional optical microscope,” said graduate student Nantao Li, the first author of the paper. “The benefit of using the photonic crystal is that it amplifies the light’s intensity so it’s easier to detect those signals and enables us to study these proteins and viruses without any chemical labels or dyes that might modify their natural state or hinder their activity – we can just use the intrinsic scattering signal as the gauge for determining if those molecules are present.” The researchers verified their technique by detecting the virus that causes COVID-19. PRISM detected individual coronaviruses as they traveled across the slide’s surface. The researchers also used PRISM to detect individual proteins such as ferritin and fibrinogen. The technique could allow researchers to study such biological targets in their natural states – watching as proteins interact, for example – or researchers could seed the surface of the photonic crystal slide with antibodies or other molecules to capture the targeted items and hold them in place. “It takes 10 seconds to get a measurement, and in that time we can count the number of viruses captured on the sensor,” Cunningham said. “It’s a single-step detection method that works at room temperature. It is also fast, very sensitive and low cost. It’s very different from the standard way we do viral testing now, which involves breaking open the viruses, extracting their genetic material and putting it through a chemical amplification process so we can detect it. That method, called PCR, is accurate and sensitive, but it requires time, specialized equipment and trained technicians.”
Researchers at the Dutch Advanced Research Center for Nanolithography (ARCNL) and Vrije Universiteit Amsterdam (VU), developed an advanced microscope capable of super-resolution microscopy through an ultra-thin fiber. Up until now, it was generally the case that the higher the resolution of a microscope, the larger the device needed to be, making it virtually impossible to look inside the human body in real-time. Although some methods that enable researchers to look inside living animals already exist, their resolution is very limited, and it takes a long time to generate an acceptable image. With the use of smart signal processing, the researchers are able to beat the theoretical limits of resolution and speed. With this newly developed compact setup, scientists are finally able to, for example, look inside the brain in real-time and high resolution, using an ultra-thin fiber. Because the method does not require any unique fluorescent labeling, it is promising for both medical uses and characterization of 3D structures in nano-lithography!
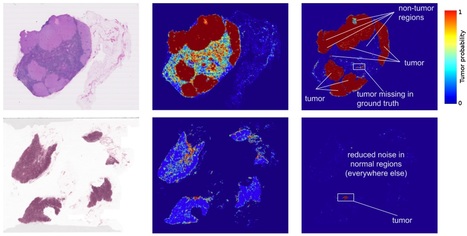
Applications of deep learning to medical disciplines including ophthalmology, dermatology, radiology, and pathology have recently shown great promise to increase both the accuracy and availability of high-quality healthcare to patients around the world. At Google, we have also published results showing that a convolutional neural network is able to detect breast cancer metastases in lymph nodes at a level of accuracy comparable to a trained pathologist. However, because direct tissue visualization using a compound light microscope remains the predominant means by which a pathologist diagnoses illness, a critical barrier to the widespread adoption of deep learning in pathology is the dependence on having a digital representation of the microscopic tissue. Using a combination of photonic time stretch and deep learning, the device is capable of analyzing 36 million images per second while leaving blood samples undamaged so they may be used for other testing purposes. The new photonic time stretch technology, invented by Barham Jalali who also led the study, takes pictures of blood cells using flashing lasers occurring in nanosecond bursts. “Each frame is slowed down in time and optically amplified so it can be digitized,” said Ata Mahjoubfar, a UCLA postdoctoral fellow who worked on the project. “This lets us perform fast cell imaging that the artificial intelligence component can distinguish.” While such a quick burst of capturing photos would typically require intense illumination, which could destroy live cells, UCLA doctoral student Clair Lifan Chen explains how Jalali’s new technique eliminates that problem. “The photonic time stretch technique allows us to identify rogue cells in a short time with low-level illumination,” Chen said. The photos are processed using deep learning, which runs data through a mass of algorithms to efficiently and accurately “read” the information. Deep learning has also been used to analyze patients’ genes, allowing identification of diseases or cancer that may otherwise go undetected, and has the potential to further understand cancer-forming mutations. So far, the newly created process has shown 95% accuracy in differentiating healthy and cancer-riddled cells — a 17% improvement over current techniques. Existent techniques for detecting cancerous cells include identifying cancer cells based on physical characteristics, a method that oftentimes misidentifies regular cells as damaged, or using fluorescent staining that binds to cancerous cells allowing cell detection yet effectively damaging the blood sample. In January 2018, a group of specialists in Wales, Germany, London, the United States, and Newcastle accomplished the same technique utilizing technology similar to face and fingerprint recognition software. In addition, the researchers found that their new method could also determine a cell’s age, which plays an important factor in the effectiveness of treatments. Continuing discoveries and advancements from scientists fighting cancer using cutting edge technology has, according to Dr. Fabian Theis of the Helmholtz Zentrum Munchen in Germany, “open[ed] a whole new perspective that could also be used for entirely different research questions, not only for cell analysis.”
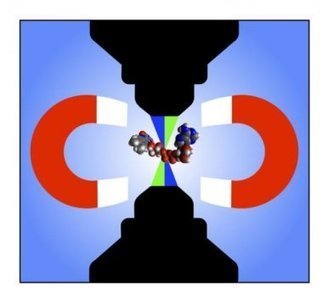
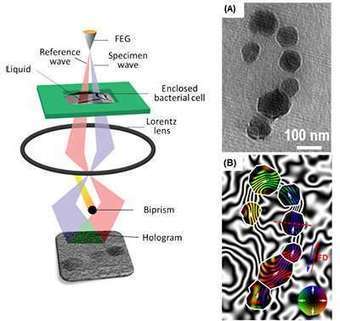
A research team led by a scientist from the U.S. Department of Energy's Ames Laboratory has demonstrated for the first time that the magnetic fields of bacterial cells and magnetic nano-objects in liquid can be studied at high resolution using electron microscopy. This proof-of-principle capability allows first-hand observation of liquid environment phenomena, and has the potential to vastly increase knowledge in a number of scientific fields, including many areas of physics, nanotechnology, biofuels conversion, biomedical engineering, catalysis, batteries and pharmacology. "It is much like being able to travel to a Jurassic Park and witness dinosaurs walking around, instead of trying to guess how they walked by examining a fossilized skeleton," said Tanya Prozorov, an associate scientist in Ames Laboratory's Division of Materials Sciences and Engineering. Prozorov works with biological and bioinspired magnetic nanomaterials, and faced what initially seemed to be an insurmountable challenge of observing them in their native liquid environment. She studies a model system, magnetotactic bacteria, which form perfect nanocrystals of magnetite. In order to best learn how bacteria do this, she needed an alternative to the typical electron microscopy process of handling solid samples in vacuum, where soft matter is studied in prepared, dried, or vitrified form. For this work, Prozorov received DOE recognition through an Office of Science Early Career Research Program grant to use cutting-edge electron microscopy techniques with a liquid cell insert to learn how the individual magnetic nanocrystals form and grow with the help of biological molecules, which is critical for making artificial magnetic nanomaterials with useful properties. To study magnetism in bacteria, she applied off-axis electron holography, a specialized technique that is used for the characterization of magnetic nanostructures in the transmission electron microscope, in combination with the liquid cell. "When we look at samples prepared in the conventional way, we have to make many assumptions about their properties based on their final state, but with the new technique, we can now observe these processes first-hand," said Prozorov. "It can help us understand the dynamics of macromolecule aggregation, nanoparticle self-assembly, and the effects of electric and magnetic fields on that process." "This method allows us to obtain large amounts of new information," said Prozorov. "It is a first step, proving that the mapping of magnetic fields in liquid at the nanometer scale with electron microscopy could be done; I am eager to see the discoveries it could foster in other areas of science."
Via Mariaschnee
Researchers at Columbia University have made a significant step toward breaking the so-called "color barrier" of light microscopy for biological systems, allowing for much more comprehensive, system-wide labeling and imaging of a greater number of biomolecules in living cells and tissues than is currently attainable. The advancement has the potential for many future applications, including helping to guide the development of therapies to treat and cure disease. In a study published online April 19 in Nature, the team, led by Associate Professor of Chemistry Wei Min, reports the development of a new optical microscopy platform with drastically enhanced detection sensitivity. Additionally, the study details the creation of new molecules that, when paired with the new instrumentation, allow for the simultaneous labeling and imaging of up to 24 specific biomolecules, nearly five times the number of biomolecules that can be imaged at the same time with existing technologies. "In the era of systems biology, how to simultaneously image a large number of molecular species inside cells with high sensitivity and specificity remains a grand challenge of optical microscopy," Min said. "What makes our work new and unique is that there are two synergistic pieces - instrumentation and molecules - working together to combat this long-standing obstacle. Our platform has the capacity to transform understanding of complex biological systems: the vast human cell map, metabolic pathways, the functions of various structures within the brain, the internal environment of tumors, and macromolecule assembly, to name just a few." All existing methods of observing a variety of structures in living cells and tissues have their own strengths, but all are also hindered by fundamental limitations, not the least of which is the existence of a "color barrier."
Via Mariaschnee
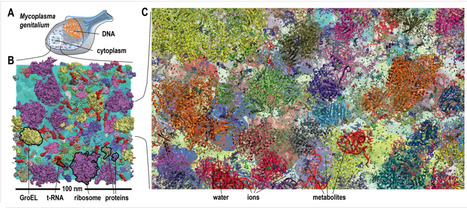
Biological macromolecules function in highly crowded cellular environments. The structure and dynamics of proteins and nucleic acids are well characterized in vitro, but in vivo crowding effects remain unclear. Using molecular dynamics simulations of a comprehensive atomistic model cytoplasm scientists found that protein-protein interactions may destabilize native protein structures, whereas metabolite interactions may induce more compact states due to electrostatic screening. Protein-protein interactions also resulted in significant variations in reduced macromolecular diffusion under crowded conditions, while metabolites exhibited significant two-dimensional surface diffusion and altered protein-ligand binding that may reduce the effective concentration of metabolites and ligands in vivo. Metabolic enzymes showed weak non-specific association in cellular environments attributed to solvation and entropic effects. These effects are expected to have broad implications for the in vivo functioning of biomolecules. This work is a first step towards physically realistic in silico whole-cell models that connect molecular with cellular biology. DOI: http://dx.doi.org/10.7554/eLife.19274.001
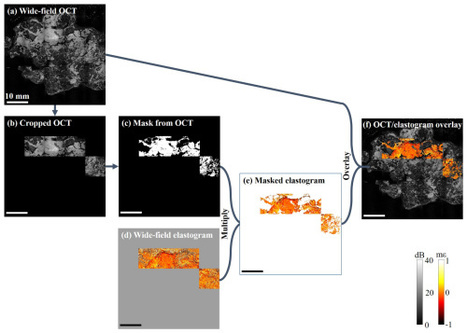
Incomplete excision of malignant tissue is a major issue in breast-conserving surgery, with typically 20 - 30% of cases requiring a second surgical procedure arising from postoperative detection of an involved margin. A team of scientists and engineers now report advances in the development of a new intraoperative tool, optical coherence micro-elastography, for the assessment of tumor margins on the micro-scale. They demonstrate an important step by conducting whole specimen imaging in intraoperative time frames with a wide-field scanning system acquiring mosaicked elastograms with overall dimensions of ~50 × 50 mm, large enough to image an entire face of most lumpectomy specimens. This capability is enabled by a wide-aperture annular actuator with an internal diameter of 65 mm. The team demonstrates feasibility by presenting elastograms recorded from freshly excised human breast tissue, including from a mastectomy, lumpectomies and a cavity shaving.
High-speed X-ray camera reveals ultrafast atomic motions at the root of organisms’ ability to turn light into biological function. Now, researchers have made a giant leap forward in taking snapshots of these ultrafast reactions in a bacterial light sensor. Using the world’s most powerful X-ray laser at the Department of Energy’s SLAC National Accelerator Laboratory, they were able to see atomic motions as fast as 100 quadrillionths of a second – 1,000 times faster than ever before. Further, “We’re the first to succeed in taking real-time snapshots of an ultrafast structure transition in a protein, in which a molecule excited by light relaxes by rearranging its structure in what is known as trans-to-cis isomerization,” says the study’s principal investigator, Marius Schmidt from the University of Wisconsin, Milwaukee. The technique could widely benefit studies of light-driven, ultrafast atomic motions. For example, it could reveal: - How visual pigments in the human eye respond to light, and how absorbing too much of it damages them.
- How photosynthetic organisms turn light into chemical energy – a process that could serve as a model for the development of new energy technologies.
- How atomic structures respond to light pulses of different shape and duration – an important first step toward controlling chemical reactions with light.
“The new data show for the first time how the bacterial sensor reacts immediately after it absorbs light,” says Andy Aquila, a researcher at SLAC’s Linac Coherent Light Source (LCLS), a DOE Office of Science User Facility. “The initial response, which is almost instantaneous, is absolutely crucial because it creates a ripple effect in the protein, setting the stage for its biological function. Only LCLS’s X-ray pulses are bright enough and short enough to capture biological processes on this ultrafast timescale.” The results were published today in Science.
Australian researchers build a world-first prototype of a new microscope that will open scientific doors.
Via Mariaschnee
A miniature handheld microscope being developed by University of Washington mechanical engineers could allow neurosurgeons to differentiate cancerous from normal brain tissue at cellular level in real time in the operating room and determine where to stop cutting.
The new technology is intended to solve a critical problem in brain surgery: to definitively distinguish between cancerous and normal brain cells, during an operation, neurosurgeons would have stop the operation and send tissue samples to a pathology lab — where they are typically frozen, sliced, stained, mounted on slides and investigated under a bulky microscope.
Developed in collaboration with Memorial Sloan Kettering Cancer Center, Stanford University and the Barrow Neurological Institute, the new microscope is outlined in an open-access paper published in January in the journalBiomedical Optics Express.
“Surgeons don’t have a very good way of knowing when they’re done cutting out a tumor,” said senior author Jonathan Liu, UW assistant professor of mechanical engineering. “They’re using their sense of sight, their sense of touch, and pre-operative images of the brain — and oftentimes it’s pretty subjective. “Being able to zoom and see at the cellular level during the surgery would really help them to accurately differentiate between tumor and normal tissues and improve patient outcomes.”
The handheld microscope, roughly the size of a pen, combines technologies in a novel way to deliver high-quality images at faster speeds than existing devices. Researchers expect to begin testing it as a cancer-screening tool in clinical settings next year.
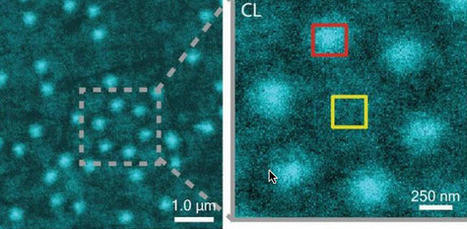
Soft matter encompasses a broad swath of materials, including liquids, polymers, gels, foam and — most importantly — biomolecules. At the heart of soft materials, governing their overall properties and capabilities, are the interactions of nano-sized components. Observing the dynamics behind these interactions is critical to understanding key biological processes, such as protein crystallization and metabolism, and could help accelerate the development of important new technologies, such as artificial photosynthesis or high-efficiency photovoltaic cells.
Observing these dynamics at sufficient resolution has been a major challenge, but this challenge is now being met with a new non-invasive nanoscale imaging technique that goes by the acronym of CLAIRE.
CLAIRE stands for “cathodoluminescence activated imaging by resonant energy transfer.” Invented by researchers with the U.S. Department of Energy (DOE)’s Lawrence Berkeley National Laboratory (Berkeley Lab) and the University of California (UC) Berkeley, CLAIRE extends the extremely high resolution of electron microscopy to the dynamic imaging of soft matter.
“Traditional electron microscopy damages soft materials and has therefore mainly been used to provide topographical or compositional information about robust inorganic solids or fixed sections of biological specimens,” says chemist Naomi Ginsberg, who leads CLAIRE’s development and holds appointments with Berkeley Lab’s Physical Biosciences Division and its Materials Sciences Division, as well as UC Berkeley’s departments of chemistry and physics.
“CLAIRE allows us to convert electron microscopy into a new non-invasive imaging modality for studying soft materials and providing spectrally specific information about them on the nanoscale.”
Ginsberg is also a member of the Kavli Energy NanoScience Institute (Kavli-ENSI) at Berkeley. She and her research group recently demonstrated CLAIRE’s imaging capabilities by applying the technique to aluminum nanostructures and polymer films that could not have been directly imaged with electron microscopy.
“What microscopic defects in molecular solids give rise to their functional optical and electronic properties? By what potentially controllable process do such solids form from their individual microscopic components, initially in the solution phase? The answers require observing the dynamics of electronic excitations or of molecules themselves as they explore spatially heterogeneous landscapes in condensed phase systems,” Ginsberg says.
“In our demonstration, we obtained optical images of aluminum nanostructures with 46 nanometer resolution, then validated the non-invasiveness of CLAIRE by imaging a conjugated polymer film. The high resolution, speed and non-invasiveness we demonstrated with CLAIRE positions us to transform our current understanding of key biomolecular interactions.”
|



 Your new post is loading...
Your new post is loading...