 Your new post is loading...
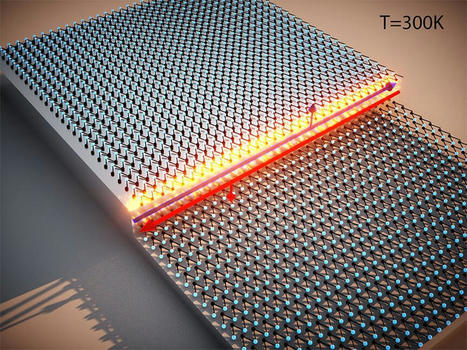
Scientists for the first time have witnessed pieces of metal crack, then fuse back together without any human intervention, overturning fundamental scientific theories in the process. If the newly discovered phenomenon can be harnessed, it could usher in an engineering revolution -- one in which self-healing engines, bridges and airplanes could reverse damage caused by wear and tear, making them safer and longer-lasting. The research team from Sandia National Laboratories and Texas A&M University described their findings today in the journal Nature. "This was absolutely stunning to watch first-hand," said Sandia materials scientist Brad Boyce. "What we have confirmed is that metals have their own intrinsic, natural ability to heal themselves, at least in the case of fatigue damage at the nanoscale," Boyce said. Fatigue damage is one way machines wear out and eventually break. Repeated stress or motion causes microscopic cracks to form. Over time, these cracks grow and spread until -- snap! The whole device breaks, or in the scientific lingo, it fails. The fissure Boyce and his team saw disappear was one of these tiny but consequential fractures -- measured in nanometers. "From solder joints in our electronic devices to our vehicle's engines to the bridges that we drive over, these structures often fail unpredictably due to cyclic loading that leads to crack initiation and eventual fracture," Boyce said. "When they do fail, we have to contend with replacement costs, lost time and, in some cases, even injuries or loss of life. The economic impact of these failures is measured in hundreds of billions of dollars every year for the U.S." Although scientists have created some self-healing materials, mostly plastics, the notion of a self-healing metal has largely been the domain of science fiction. "Cracks in metals were only ever expected to get bigger, not smaller. Even some of the basic equations we use to describe crack growth preclude the possibility of such healing processes," Boyce said. Unexpected discovery confirmed by theory's originator In 2013, Michael Demkowicz -- then an assistant professor at the Massachusetts Institute of Technology's department of materials science and engineering, now a full professor at Texas A&M -- began chipping away at conventional materials theory. He published a new theory, based on findings in computer simulations, that under certain conditions metal should be able to weld shut cracks formed by wear and tear. The discovery that his theory was true came inadvertently at the Center for Integrated Nanotechnologies, a Department of Energy user facility jointly operated by Sandia and Los Alamos national laboratories. "We certainly weren't looking for it," Boyce said. Khalid Hattar, now an associate professor at the University of Tennessee, Knoxville, and Chris Barr, who now works for the Department of Energy's Office of Nuclear Energy, were running the experiment at Sandia when the discovery was made. They only meant to evaluate how cracks formed and spread through a nanoscale piece of platinum using a specialized electron microscope technique they had developed to repeatedly pull on the ends of the metal 200 times per second.
The arrangement of electrons in matter, known as the electronic structure, plays a crucial role in fundamental but also applied research, such as drug design and energy storage. However, the lack of a simulation technique that offers both high fidelity and scalability across different time and length scales has long been a roadblock for the progress of these technologies. Researchers from the Center for Advanced Systems Understanding (CASUS) at the Helmholtz-Zentrum Dresden-Rossendorf (HZDR) in Görlitz, Germany, and Sandia National Laboratories in Albuquerque, New Mexico, U.S., have now pioneered a machine learning–based simulation method that supersedes traditional electronic structure simulation techniques. Their Materials Learning Algorithms (MALA) software stack enables access to previously unattainable length scales. The work is published in the journal npj Computational Materials. Electrons are elementary particles of fundamental importance. Their quantum mechanical interactions with one another and with atomic nuclei give rise to a multitude of phenomena observed in chemistry and materials science. Understanding and controlling the electronic structure of matter provides insights into the reactivity of molecules, the structure and energy transport within planets, and the mechanisms of material failure. Scientific challenges are increasingly being addressed through computational modeling and simulation, leveraging the capabilities of high-performance computing. However, a significant obstacle to achieving realistic simulations with quantum precision is the lack of a predictive modeling technique that combines high accuracy with scalability across different length and time scales. Classical atomistic simulation methods can handle large and complex systems, but their omission of quantum electronic structure restricts their applicability. Conversely, simulation methods which do not rely on assumptions such as empirical modeling and parameter fitting (first principles methods) provide high fidelity but are computationally demanding. For instance, density functional theory (DFT), a widely used first principles method, exhibits cubic scaling with system size, thus restricting its predictive capabilities to small scales. Hybrid approach based on deep learning The team of researchers now presented a novel simulation method called the Materials Learning Algorithms (MALA) software stack. In computer science, a software stack is a collection of algorithms and software components that are combined to create a software application for solving a particular problem. Lenz Fiedler, a Ph.D. student and key developer of MALA at CASUS, explains, "MALA integrates machine learning with physics-based approaches to predict the electronic structure of materials. It employs a hybrid approach, utilizing an established machine learning method called deep learning to accurately predict local quantities, complemented by physics algorithms for computing global quantities of interest."
The cold blast of an air conditioner can be a welcome relief as temperatures soar, but A/C units require large amounts of energy and can leak potent greenhouse gases. Today, scientists report an eco-friendly alternative—a plant-based film that gets cooler when exposed to sunlight and comes in a variety of textures and bright, iridescent colors. The material could someday keep buildings, cars and other structures cool without requiring external power. The researchers will present their results at the spring meeting of the American Chemical Society (ACS). ACS Spring 2023 is a hybrid meeting being held virtually and in-person March 26–30 2023. "To make materials that remain cooler than the air around them during the day, you need something that reflects a lot of solar light and doesn't absorb it, which would transform energy from the light into heat," says Silvia Vignolini, Ph.D., the project's principal investigator. "There are only a few materials that have this property, and adding color pigments would typically undo their cooling effects," Vignolini adds. Passive daytime radiative cooling (PDRC) is the ability of a surface to emit its own heat into space without it being absorbed by the air or atmosphere. The result is a surface that, without using any electrical power, can become several degrees colder than the air around it. When used on buildings or other structures, materials that promote this effect can help limit the use of air conditioning and other power-intensive cooling methods. Some paints and films currently in development can achieve PDRC, but most of them are white or have a mirrored finish, says Qingchen Shen, Ph.D., who is presenting the work at the meeting. Both Vignolini and Shen are at Cambridge University (U.K.). But a building owner who wanted to use a blue-colored PDRC paint would be out of luck—colored pigments, by definition, absorb specific wavelengths of sunlight and only reflect the colors we see, causing undesirable warming effects in the process. But there's a way to achieve color without the use of pigments. Soap bubbles, for example, show a prism of different colors on their surfaces. These colors result from the way light interacts with differing thicknesses of the bubble's film, a phenomenon called structural color. Part of Vignolini's research focuses on identifying the causes behind different types of structural colors in nature. In one case, her group found that cellulose nanocrystals (CNCs), which are derived from the cellulose found in plants, could be made into iridescent, colorful films without any added pigment. As it turns out, cellulose is also one of the few naturally occurring materials that can promote PDRC. Vignolini learned this after hearing a talk from the first researchers to have created a cooling film material. "I thought wow, this is really amazing, and I never really thought cellulose could do this." In recent work, Shen and Vignolini layered colorful CNC materials with a white-colored material made from ethyl cellulose, producing a colorful bi-layered PDRC film. They made films with vibrant blue, green and red colors that, when placed under sunlight, were an average of nearly 40 F cooler than the surrounding air. A square meter of the film generated over 120 Watts of cooling power, rivaling many types of residential air conditioners. The most challenging aspect of this research, Shen says, was finding a way to make the two layers stick together—on their own, the CNC films were brittle, and the ethyl cellulose layer had to be plasma-treated to get good adhesion. The result, however, was films that were robust and could be prepared several meters at a time in a standard manufacturing line.
Laser powder bed fusion, a 3D-printing technique, offers potential in the manufacturing industry, particularly when fabricating nickel-titanium shape memory alloys with complex geometries. Although this manufacturing technique is attractive for applications in the biomedical and aerospace fields, it has rarely showcased the superelasticity required for specific applications using nickel-titanium shape memory alloys. Defects generated and changes imposed onto the material during the 3D-printing process prevented the superelasticity from appearing in 3D-printed nickel-titanium. Researchers from Texas A&M University recently showcased superior tensile superelasticity by fabricating a shape memory alloy through laser powder bed fusion, nearly doubling the maximum superelasticity reported in literature for 3D printing. This study was recently published in vol. 229 of the Acta Materialia journal. Nickel-titanium shape memory alloys have various applications due to their ability to return to their original shape upon heating or upon removal of the applied stress. Therefore, they can be used in biomedical and aerospace fields for stents, implants, surgical devices and aircraft wings. However, developing and properly fabricating these materials requires extensive research to characterize functional properties and examine the microstructure. "Shape memory alloys are smart materials that can remember their high-temperature shapes," said Dr. Lei Xue, a former doctoral student in the Department of Materials Science and Engineering and the first author of the publication. "Although they can be utilized in many ways, fabricating shape memory alloys into complex shapes requires fine-tuning to ensure the material exhibits the desired properties."
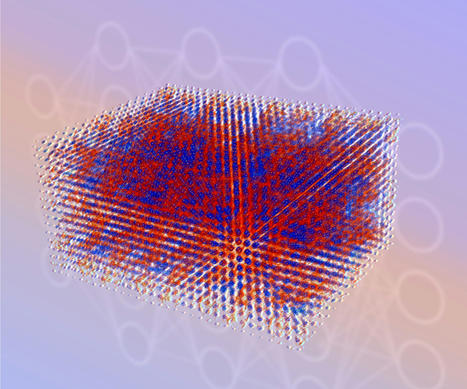
Scientists with the University of Chicago have discovered a way to create a material that can be made like a plastic, but conducts electricity more like a metal. The research, published Oct. 26th 2022 in Nature, shows how to make a kind of material in which the molecular fragments are jumbled and disordered, but can still conduct electricity extremely well. This goes against all of the rules we know about for conductivity -- to a scientist, it's kind of seeing a car driving on water and still going 70 mph. But the finding could also be extraordinarily useful; if you want to invent something revolutionary, the process often first starts with discovering a completely new material. "In principle, this opens up the design of a whole new class of materials that conduct electricity, are easy to shape, and are very robust in everyday conditions," said John Anderson, an associate professor of chemistry at the University of Chicago and the senior author on the study. "Essentially, it suggests new possibilities for an extremely important technological group of materials," said Jiaze Xie (PhD'22, now at Princeton), the first author on the paper. 'There isn't a solid theory to explain this' Conductive materials are absolutely essential if you're making any kind of electronic device, whether it be an iPhone, a solar panel, or a television. By far the oldest and largest group of conductors is the metals: copper, gold, aluminum. Then, about 50 years ago, scientists were able to create conductors made out of organic materials, using a chemical treatment known as "doping," which sprinkles in different atoms or electrons through the material. This is advantageous because these materials are more flexible and easier to process than traditional metals, but the trouble is they aren't very stable; they can lose their conductivity if exposed to moisture or if the temperature gets too high. But fundamentally, both of these organic and traditional metallic conductors share a common characteristic. They are made up of straight, closely packed rows of atoms or molecules. This means that electrons can easily flow through the material, much like cars on a highway. In fact, scientists thought a material had to have these straight, orderly rows in order to conduct electricity efficiently. Then Xie began experimenting with some materials discovered years ago, but largely ignored. He strung nickel atoms like pearls into a string of of molecular beads made of carbon and sulfur, and began testing. To the scientists' astonishment, the material easily and strongly conducted electricity. What's more, it was very stable. "We heated it, chilled it, exposed it to air and humidity, and even dripped acid and base on it, and nothing happened," said Xie. That is enormously helpful for a device that has to function in the real world. But to the scientists, the most striking thing was that the molecular structure of the material was disordered. "From a fundamental picture, that should not be able to be a metal," said Anderson. "There isn't any solid theory to explain this."
For the first time, physicists have observed novel quantum effects in a topological insulator at room temperature. This breakthrough, published as the cover article of the October issue of Nature Materials, came when Princeton scientists explored a topological material based on the element bismuth. The scientists have used topological insulators to demonstrate quantum effects for more than a decade, but this experiment is the first time these effects have been observed at room temperature. Typically, inducing and observing quantum states in topological insulators requires temperatures around absolute zero, which is equal to -459 degrees Fahrenheit (or -273 degrees Celsius). This finding opens up a new range of possibilities for the development of efficient quantum technologies, such as spin-based electronics, which may potentially replace many current electronic systems for higher energy efficiency. In recent years, the study of topological states of matter has attracted considerable attention among physicists and engineers and is presently the focus of much international interest and research. This area of study combines quantum physics with topology—a branch of theoretical mathematics that explores geometric properties that can be deformed but not intrinsically changed. "The novel topological properties of matter have emerged as one of the most sought-after treasures in modern physics, both from a fundamental physics point of view and for finding potential applications in next-generation quantum engineering and nanotechnologies," said M. Zahid Hasan, the Eugene Higgins Professor of Physics at Princeton University, who led the research.
An international collaboration of scientists has created and observed an entirely new class of vortices -- the whirling masses of fluid or air. Led by researchers from Amherst College in the US and the University of East Anglia and Lancaster University in the UK, their new paper details the first laboratory studies of these 'exotic' whirlpools in an ultracold gas of atoms at temperatures as low as tens of billionths of a degree above absolute zero. The discovery, announced this week in the journal Nature Communications, may have exciting future implications for implementations of quantum information and computing. Vortices are familiar objects in nature, from the whirlpools of water down a bathtub drain to the airflow around a hurricane. In quantum-mechanical systems, such as an atomic Bose-Einstein condensate, the vortices tend to be tiny and their circulation comes in discrete, quantized units. Such vortices have long been objects of fascination for physicists and have helped to illuminate the unusual properties of superfluidity and superconductivity. The unusual nature of the observed whirlpools here, however, is due to symmetries in the quantum gas. One especially fascinating property of physical theories, from cosmology to elementary particles, is the appearance of asymmetric worlds despite perfect underlying symmetries. For example, when water freezes to ice, disordered molecules in a liquid arrange themselves into a periodic array. The spatial symmetry of a system is often readily identified -- for example, a honeycomb has a periodic array of cells with hexagonal symmetry. Although the vortex medium used in this new work is a fluid rather than a solid array, it also possesses an internal set of hidden discrete symmetries. For example, one of the team's ultracold gases had the fourfold symmetry of a square, and another had the tetrahedral symmetry of a four-sided die, familiar to players of fantasy games everywhere. "The mass flow and the underlying symmetry of the fluid interact with one another in interesting ways," said Dr Magnus Borgh, Associate Professor in Physics at UEA. "One consequence is that if the positions of two vortices are interchanged, they can leave a trace of the process lingering in the fluid. This trace links the interacting vortices together permanently, like a rung in a ladder. No ordinary fluids behave like this, and it may be that analogous objects only exist deep inside neutron stars," added Prof Janne Ruostekoski, of Lancaster University. Indeed, the team says these created vortices go beyond the state-of-the-art.
MIT physicsts identified new multilayered configurations of graphene that can be twisted and stacked to elicit robust superconductivity at low temperatures. The study establishes these configurations as the first known “family” of multilayer magic-angle superconductors. When it comes to graphene, it appears that superconductivity runs in the family. Graphene is a single-atom-thin material that can be exfoliated from the same graphite that is found in pencil lead. The ultrathin material is made entirely from carbon atoms that are arranged in a simple hexagonal pattern, similar to that of chicken wire. Since its isolation in 2004, graphene has been found to embody numerous remarkable properties in its single-layer form. In 2018, MIT researchers found that if two graphene layers are stacked at a very specific “magic” angle, the twisted bilayer structure could exhibit robust superconductivity, a widely sought material state in which an electrical current can flow through with zero energy loss. Recently, the same group found a similar superconductive state exists in twisted trilayer graphene — a structure made from three graphene layers stacked at a precise, new magic angle. Now the team reports that — you guessed it — four and five graphene layers can be twisted and stacked at new magic angles to elicit robust superconductivity at low temperatures. This latest discovery, published this week in Nature Materials, establishes the various twisted and stacked configurations of graphene as the first known “family” of multilayer magic-angle superconductors. The team also identified similarities and differences between graphene family members. The findings could serve as a blueprint for designing practical, room-temperature superconductors. If the properties among family members could be replicated in other, naturally conductive materials, they could be harnessed, for instance, to deliver electricity without dissipation or build magnetically levitating trains that run without friction. “The magic-angle graphene system is now a legitimate ‘family,’ beyond a couple of systems,” says lead author Jeong Min (Jane) Park, a graduate student in MIT’s Department of Physics. “Having this family is particularly meaningful because it provides a way to design robust superconductors.”
Geckos are famous for having grippy feet that allow them to scale vertical surfaces with ease. They get this seeming superpower from millions of microscopic, hairlike structures on their toes. Now, scientists have zoomed in for an even closer look at those structures, called setae, and found that they are coated in an ultra-thin film of water-repelling lipid molecules only one nanometer, or billionths of a meter, thick. Researchers from the National Institute of Standards and Technology (NIST) analyzed the surface of the setae using high-energy X-rays thrown off by a type of particle accelerator called a synchrotron. The synchrotron microscope showed that the lipid molecules line the surface of the setae in dense, orderly arrays. Lipids can play a role in this process because they are hydrophobic, meaning they repel water. "The lipids might function to push away any water beneath the spatulae, allowing them to make closer contact with the surface," said physicist and co-author Tobias Weidner of Aarhus University in Denmark. "This would help geckos maintain their grip on wet surfaces." The setae and spatulae are made of a type of keratin protein similar to that found in human hair and fingernails. They are extremely delicate. The researchers showed that the keratin fibers are aligned in the direction of the setae, which might help them resist abrasion. "The most exciting thing for me about this biological system is that everything is perfectly optimized on every scale, from the macro to the micro to the molecular," said biologist and co-author Stanislav Gorb of Kiel University in Germany. "This can help biomimetic engineers know what to do next. You can imagine gecko boots that don't slip on wet surfaces, or gecko gloves for holding tools that are wet," said NIST physicist and co-author Dan Fischer. "Or a vehicle that can run up walls, or a robot that can run along power lines and inspect them." The NIST synchrotron microscope that the researchers used to analyze the setae is unique in its ability to identify molecules on the surface of a three-dimensional object, measure their orientation and map their position. It is located at the U.S. Department of Energy's Brookhaven National Laboratory, where the National Synchrotron Light Source II, a half-mile-long particle accelerator, provides a source of high-energy X-rays for illumination. This microscope is typically used to understand the physics of advanced industrial materials, including batteries, semiconductors, solar panels and medical devices. "But it is fascinating to figure out how gecko feet work," Fischer said, "and we can learn a lot from nature when it comes to improving our own technology."
A new database and searchable tool reveals more than 90,000 known materials with electronic properties that remain unperturbed in the face of disruption. Topology stems from a branch of mathematics that studies shapes that can be manipulated or deformed without losing certain core properties. A donut is a common example: If it were made of rubber, a donut could be twisted and squeezed into a completely new shape, such as a coffee mug, while retaining a key trait — namely, its center hole, which takes the form of the cup’s handle. The hole, in this case, is a topological trait, robust against certain deformations. In recent years, scientists have applied concepts of topology to the discovery of materials with similarly robust electronic properties. In 2007, researchers predicted the first electronic topological insulators — materials in which electrons that behave in ways that are “topologically protected,” or persistent in the face of certain disruptions. Since then, scientists have searched for more topological materials with the aim of building better, more robust electronic devices. Until recently, only a handful of such materials were identified, and were therefore assumed to be a rarity. Now researchers at MIT and elsewhere have discovered that, in fact, topological materials are everywhere, if you know how to look for them. In a paper published today in Science, the team, led by Nicolas Regnault of Princeton University and the École Normale Supérieure Paris, reports harnessing the power of multiple supercomputers to map the electronic structure of more than 96,000 natural and synthetic crystalline materials. They applied sophisticated filters to determine whether and what kind of topological traits exist in each structure. Overall, they found that 90 percent of all known crystalline structures contain at least one topological property, and more than 50 percent of all naturally occurring materials exhibit some sort of topological behavior. “We found there’s a ubiquity — topology is everywhere,” says Benjamin Wieder, the study’s co-lead, and a postdoc in MIT’s Department of Physics. The team has compiled the newly identified materials into a new, freely accessible Topological Materials Database resembling a periodic table of topology. With this new library, scientists can quickly search materials of interest for any topological properties they might hold, and harness them to build ultra-low-power transistors, new magnetic memory storage, and other devices with robust electronic properties.
A team of researchers from the University of Massachusetts Amherst recently announced in the Proceedings of the National Academy of Sciences that they had engineered a new rubber-like solid substance that has surprising qualities. It can absorb and release very large quantities of energy. And it is programmable. Taken together, this new material holds great promise for a very wide array of applications, from enabling robots to have more power without using additional energy, to new helmets and protective materials that can dissipate energy much more quickly. "Imagine a rubber band," says Alfred Crosby, professor of polymer science and engineering at UMass Amherst and the paper's senior author. "You pull it back, and when you let it go, it flies across the room. Now imagine a super rubber band. When you stretch it past a certain point, you activate extra energy stored in the material. When you let this rubber band go, it flies for a mile." This hypothetical rubber band is made out of a new metamaterial -- a substance engineered to have a property not found in naturally occurring materials -- that combines an elastic, rubber-like substance with tiny magnets embedded in it. This new "elasto-magnetic" material takes advantage of a physical property known as a phase shift to greatly amplify the amount of energy the material can release or absorb. A phase shift occurs when a material moves from one state to another: think of water turning into steam or liquid concrete hardening into a sidewalk. Whenever a material shifts its phase, energy is either released or absorbed. And phase shifts aren't just limited to changes between liquid, solid and gaseous states -- a shift can occur from one solid phase to another. A phase shift that releases energy can be harnessed as a power source, but getting enough energy has always been the difficult part. "To amplify energy release or absorption, you have to engineer a new structure at the molecular or even atomic level," says Crosby. However, this is challenging to do and even more difficult to do in a predictable way. But by using metamaterials, Crosby says that "we have overcome these challenges, and have not only made new materials, but also developed the design algorithms that allow these materials to be programmed with specific responses, making them predictable." The team has been inspired by some of the lightning-quick responses seen in nature: the snapping-shut of Venus flytraps and trap-jaw ants. "We've taken this to the next level," says Xudong Liang, the paper's lead author, currently a professor at Harbin Institute of Technology, Shenzhen (HITSZ) in China who completed this research while a postdoc at UMass Amherst. "By embedding tiny magnets into the elastic material, we can control the phase transitions of this metamaterial. And because the phase shift is predictable and repeatable, we can engineer the metamaterial to do exactly what we want it to do: either absorbing the energy from a large impact, or releasing great quantities of energy for explosive movement."
University of Texas at Austin researchers have created a new sodium-based battery material that is highly stable, capable of recharging as quickly as a traditional lithium-ion battery and able to pave the way toward delivering more energy than current battery technologies. For about a decade, scientists and engineers have been developing sodium batteries, which replace both lithium and cobalt used in current lithium-ion batteries with cheaper, more environmentally friendly sodium. Unfortunately, in earlier sodium batteries, a component called the anode would tend to grow needle-like filaments called dendrites that can cause the battery to electrically short and even catch fire or explode. In one of two recent sodium battery advances from UT Austin, the new material solves the dendrite problem and recharges as quickly as a lithium-ion battery. The team published their results in the journal Advanced Materials. "We're essentially solving two problems at once," said David Mitlin, a professor in the Cockrell School of Engineering's Walker Department of Mechanical Engineering and Applied Research Laboratory who designed the new material. "Typically, the faster you charge, the more of these dendrites you grow. So if you suppress dendrite growth, you can charge and discharge faster, because all of a sudden it's safe." Graeme Henkelman, a professor in the Department of Chemistry and the Oden Institute for Computational Engineering and Sciences, used a computer model to explain, from a theoretical perspective, why the material has the unique properties it does. "This material is also exciting because the sodium metal anode theoretically has the highest energy density of any sodium anode," Henkelman said.
The central principle of superconductivity is that electrons form pairs. But can they also condense into foursomes? Recent findings have suggested they can, and a physicist at KTH Royal Institute of Technology today published the first experimental evidence of this quadrupling effect and the mechanism by which this state of matter occurs.
Reporting today in Nature Physics, Professor Egor Babaev and collaborators presented evidence of fermion quadrupling in a series of experimental measurements on the iron-based material, Ba1−xKxFe2As2. The results follow nearly 20 years after Babaev first predicted this kind of phenomenon, and eight years after he published a paper predicting that it could occur in the material.
The pairing of electrons enables the quantum state of superconductivity, a zero-resistance state of conductivity which is used in MRI scanners and quantum computing. It occurs within a material as a result of two electrons bonding rather than repelling each other, as they would in a vacuum. The phenomenon was first described in a theory by, Leon Cooper, John Bardeen and John Schrieffer, whose work was awarded the Nobel Prize in 1972.
So-called Cooper pairs are basically “opposites that attract”. Normally two electrons, which are negatively-charged subatomic particles, would strongly repel each other. But at low temperatures in a crystal they become loosely bound in pairs, giving rise to a robust long-range order. Currents of electron pairs no longer scatter from defects and obstacles and a conductor can lose all electrical resistance, becoming a new state of matter: a superconductor.
Only in recent years has the theoretical idea of four-fermion condensates become broadly accepted.
For a fermion quadrupling state to occur there has to be something that prevents condensation of pairs and prevents their flow without resistance, while allowing condensation of four-electron composites, Babaev says.
The Bardeen-Cooper-Schrieffer theory didn’t allow for such behavior, so when Babaev’s experimental collaborator at Technische Universtät Dresden, Vadim Grinenko, found in 2018 the first signs of a fermion quadrupling condensate, it challenged years of prevalent scientific agreement.
|
While paper isn’t exactly a smart material, it someday could be if it is covered in a new type of liquid metal. This liquid alloy has the potential to turn paper and other materials into gadgets that can do some things on their own. Liquid metal is already used in smart objects like circuits and wearable sensors—but not as a coating. Inspired by origami, a team of scientists led by Bo Yuan of Tsinghua University in China has figured out a way to formulate liquid metal and apply it with a stamp so it sticks to paper without an adhesive, which has never been possible before. In a study recently published in Cell Reports Physical Science, the scientists showed that paper coated in the metal can be crafted into origami shapes and re-fold itself. The metal coating also conducts heat and electricity. It’s like magic. Almost. A sticky alloy Because the particles in liquid metal tend to stay so close together, it is difficult to get it to adhere to any surface without something that acts as glue. But these adhesives usually have a negative effect on the metal’s properties, such as its conductivity. Yuan and his team wanted a liquid metal that could stick to paper without an adhesive. They used an alloy of bismuth, indium, and tin oxide (BiInSn) and tested how well it performed next to an indium/gallium alloy (eGaIn). BiInSn turned out to be more effective. Unlike eGaIn, it doesn’t oxidize when exposed to air, so how well it sticks to a surface does not depend on the oxide film that forms on the metal. BiInSn is a solid at room temperature and has a higher melting point, so there is no risk of it liquefying at temperatures under 62° Celsius (about 144° Fahrenheit). It is also capable of stronger adhesion. However, getting optimal adhesion out of BiInSn required trial and error. “We needed to ensure the adhesion of liquid metal to be uniform in large scale on different paper, and to maintain the stability of the coating,” Yuan told Ars Technica in an email interview. “To solve these problems, we changed pressure applied on the stamp as well as the rubbing speed used in the experiments and finally found the most suitable parameters, which finally achieved fast, large-scale, and stable adhesion.” The researchers tried stamping it onto paper with different amounts of pressure and found out that not much is needed for it to stay in place. They then created an origami cube out of the metal-coated paper, which required the edges to adhere to each other without any other binding agent. They even saw that when that square was unfolded, the coated paper could fold itself back into its original shape. Because the metal coating was self-adhesive, the edges that had been unfolded attracted each other until the paper became a cube again. Another shape they tried was a spring that could be stretched or compressed and would remain however it was adjusted. It was also possible for the team to build 3D structures out of individual pieces of flat, metal-coated paper. These structures could keep their shape without falling apart, and the coating could just be peeled off afterward without affecting the properties of its paper substrate in any way. The coating, which also lost none of its properties, could be recycled and used repeatedly. The paper just went back to being paper. Next steps Yuan thinks self-adhesion through liquid metal is an advantage, because, if it can be done with paper, it could be done with other thin, lightweight materials to create smart objects and soft robots that can fit into tight spaces. The next thing he wants to accomplish is finding a coating where the metal does not peel off once solidified. He is considering testing bio-friendly paint spray to protect the coating in materials that may eventually be used as packaging (boxes could open and close themselves just like the paper cube in the experiment), on human skin (bandages would come off painlessly without glue), underwater, and even in conditions seen on other planets and moons. This substance could possibly be an asset to soft robots in alien environments. Some soft robots can already explore the deepest reaches of the ocean where the pressure is too high for humans and the cracks and crevices too small for larger machines. Soft robots are being designed with an eye for subsurface tunnels on Mars and other bodies in space. Autonomous soft robots that are thin and flexible would be able to venture into places where larger rovers are unable to fit or navigate safely, and the self-adhesion of the liquid metal coating would allow them to fold and unfold on their own. “Utilizing our method, one can quickly create smart materials with good thermal and electrical conductivity as well as stiffness-tunable ability, which greatly expands material options for soft robots,” Yuan said in the interview. “I think that this method may provide a new route for designing space explorers." Cell Reports Physical Science, 2023. DOI: 10.1016/j.xcrp.2023.101419 (About DOIs).
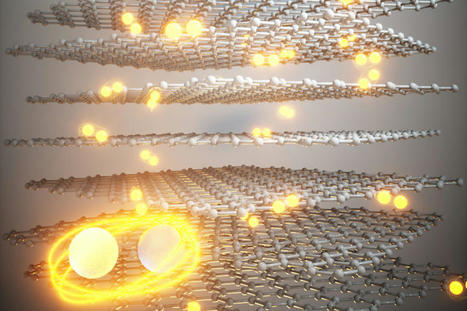
Catalyst materials are critical for refining petroleum products and for manufacturing pharmaceuticals, plastics, food additives, fertilizers, green fuels, industrial chemicals and much more. Catalyst materials accelerate chemical reactions without undergoing changes themselves. They are critical for refining petroleum products and for manufacturing pharmaceuticals, plastics, food additives, fertilizers, green fuels, industrial chemicals and much more. Scientists and engineers have spent decades fine-tuning catalytic reactions -- yet because it's currently impossible to directly observe those reactions at the extreme temperatures and pressures often involved in industrial-scale catalysis, they haven't known exactly what is taking place on the nano and atomic scales. This new research helps unravel that mystery with potentially major ramifications for industry. In fact, just three catalytic reactions -- steam-methane reforming to produce hydrogen, ammonia synthesis to produce fertilizer, and methanol synthesis -- use close to 10% of the world's energy. "If you decrease the temperatures at which you have to run these reactions by only a few degrees, there will be an enormous decrease in the energy demand that we face as humanity today," says Manos Mavrikakis, a professor of chemical and biological engineering at UW-Madison who led the research. "By decreasing the energy needs to run all these processes, you are also decreasing their environmental footprint." In their research, the UW-Madison engineers develop and use powerful modeling techniques to simulate catalytic reactions at the atomic scale. For this study, they looked at reactions involving transition metal catalysts in nanoparticle form, which include elements like platinum, palladium, rhodium, copper, nickel, and others important in industry and green energy. According to the current rigid-surface model of catalysis, the tightly packed atoms of transition metal catalysts provide a 2D surface that chemical reactants adhere to and participate in reactions. When enough pressure and heat or electricity is applied, the bonds between atoms in the chemical reactants break, allowing the fragments to recombine into new chemical products. "The prevailing assumption is that these metal atoms are strongly bonded to each other and simply provide 'landing spots' for reactants. What everybody has assumed is that metal-metal bonds remain intact during the reactions they catalyze," says Mavrikakis. "So here, for the first time, we asked the question, 'Could the energy to break bonds in reactants be of similar amounts to the energy needed to disrupt bonds within the catalyst?'" According to Mavrikakis's modeling, the answer is yes. The energy provided for many catalytic processes to take place is enough to break bonds and allow single metal atoms (known as adatoms) to pop loose and start traveling on the surface of the catalyst. These adatoms combine into clusters, which serve as sites on the catalyst where chemical reactions can take place much easier than the original rigid surface of the catalyst. Using a set of special calculations, the team looked at industrially important interactions of eight transition metal catalysts and 18 reactants, identifying energy levels and temperatures likely to form such small metal clusters, as well as the number of atoms in each cluster, which can also dramatically affect reaction rates. Their experimental collaborators at the University of California, Berkeley, used atomically-resolved scanning tunneling microscopy to look at carbon monoxide adsorption on nickel (111), a stable, crystalline form of nickel useful in catalysis. Their experiments confirmed models that showed various defects in the structure of the catalyst can also influence how single metal atoms pop loose, as well as how reaction sites form. Mavrikakis says the new framework is challenging the foundation of how researchers understand catalysis and how it takes place. It may apply to other non-metal catalysts as well, which he will investigate in future work. It is also relevant to understanding other important phenomena, including corrosion and tribology, or the interaction of surfaces in motion. "We're revisiting some very well-established assumptions in understanding how catalysts work and, more generally, how molecules interact with solids," Mavrikakis says.
Cavities concentrate light and enhance its interaction with matter. Confining to microscopic volumes is necessary for many applications but space constraints in such cavities limit the design freedom. A team of scientists now demonstrated stable optical microcavities by counteracting the phase evolution of the cavity modes using an amorphous Silicon metasurface as cavity end mirror. Careful design allowed them to limit the metasurface scattering losses at telecom wavelengths to less than 2% and using a distributed Bragg reflector as metasurface substrate ensures high reflectivity. This demonstration experimentally achieves telecom-wavelength microcavities with quality factors of up to 4600, spectral resonance linewidths below 0.4 nm, and very small mode volumes. The method introduces freedom to stabilize modes with arbitrary transverse intensity profiles and to design cavity-enhanced hologram modes. This approach introduces the nanoscopic light control capabilities of dielectric metasurfaces to cavity electrodynamics and is industrially scalable using semiconductor manufacturing processes.
A new type of material can learn and improve its ability to deal with unexpected forces thanks to a unique lattice structure with connections of variable stiffness, as described in a new paper. The new material is a type of architected material, which gets its properties mainly from the geometry and specific traits of its design rather than what it is made out of. Take hook-and-loop fabric closures like Velcro, for example. It doesn’t matter whether it is made from cotton, plastic or any other substance. As long as one side is a fabric with stiff hooks and the other side has fluffy loops, the material will have the sticky properties of Velcro. The new material’s architecture is based on that of an artificial neural network—layers of interconnected nodes that can learn to do tasks by changing how much importance, or weight, they place on each connection. Theoretically, such a mechanical lattice with physical nodes could be trained to take on certain mechanical properties by adjusting each connection’s rigidity. To find out if a mechanical lattice indeed would be able to adopt and maintain new properties—like taking on a new shape or changing directional strength—scientists started off by building a computer model. They then selected a desired shape for the material as well as input forces and had a computer algorithm tune the tensions of the connections so that the input forces would produce the desired shape. They did this training on 200 different lattice structures and found that a triangular lattice was best at achieving all of the shapes tested. Once the many connections are tuned to achieve a set of tasks, the material will continue to react in the desired way. The training is—in a sense—remembered in the structure of the material itself. The researchers then built a physical prototype lattice with adjustable electromechanical springs arranged in a triangular lattice. The prototype is made of 6-inch connections and is about 2 feet long by 1½ feet wide. And it worked beautifully. When the lattice and algorithm worked together, the material was able to learn and change shape in particular ways when subjected to different forces. The scientists call this new material a mechanical neural network.
MIT researchers have developed a technique for precisely controlling the arrangement and placement of nanoparticles on a material, like the silicon used for computer chips, in a way that does not damage or contaminate the surface of the material. The technique, which combines chemistry and directed assembly processes with conventional fabrication techniques, enables the efficient formation of high-resolution, nanoscale features integrated with nanoparticles for devices like sensors, lasers, and LEDs, which could boost their performance. Transistors and other nanoscale devices are typically fabricated from the top down—materials are etched away to reach the desired arrangement of nanostructures. But creating the smallest nanostructures, which can enable the highest performance and new functionalities, requires expensive equipment and remains difficult to do at scale and with the desired resolution. A more precise way to assemble nanoscale devices is from the bottom up. In one scheme, engineers have used chemistry to "grow" nanoparticles in solution, drop that solution onto a template, arrange the nanoparticles, and then transfer them to a surface. However, this technique also involves steep challenges. First, thousands of nanoparticles must be arranged on the template efficiently. And transferring them to a surface typically requires a chemical glue, large pressure, or high temperatures, which could damage the surfaces and the resulting device. The MIT researchers developed a new approach to overcome these limitations. They used the powerful forces that exist at the nanoscale to efficiently arrange particles in a desired pattern and then transfer them to a surface without any chemicals or high pressures, and at lower temperatures. Because the surface material remains pristine, these nanoscale structures can be incorporated into components for electronic and optical devices, where even minuscule imperfections can hamper performance. "This approach allows you, through engineering of forces, to place the nanoparticles, despite their very small size, in deterministic arrangements with single-particle resolution and on diverse surfaces, to create libraries of nanoscale building blocks that can have very unique properties, whether it is their light-matter interactions, electronic properties, mechanical performance, etc.," says Farnaz Niroui, the EE Landsman Career Development Assistant Professor of Electrical Engineering and Computer Science (EECS) at MIT. "By integrating these building blocks with other nanostructures and materials we can then achieve devices with unique functionalities that would not be readily feasible to make if we were to use the conventional top-down fabrication strategies alone."
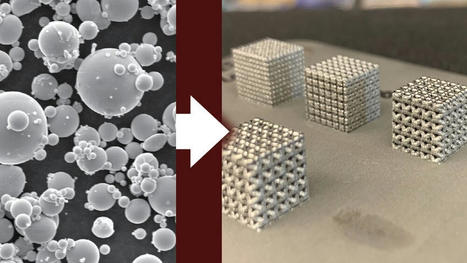
In a recent study, researchers from Cambridge investigated a promising alternative called tetrataenite. This mineral is an alloy of iron and nickel arranged in a stacked crystalline structure, which gives it magnetic properties similar to those of rare earth magnets. The advantage of course is that iron and nickel are much easier to come by.
The problem is, tetrataenite is tricky to find – it mostly shows up in meteorite samples, where it’s thought to have taken millions of years to form. Previous attempts to produce it artificially in the lab have shown some success, but the techniques aren’t scalable.
On closer inspection of meteorite samples of tetrataenite, the team found that phosphorus was in the mix, helping to speed up the arrangement of the iron and nickel atoms into the stack structure. So, they mixed iron, nickel and phosphorus together in specific quantities, and found that tetrataenite formed up to 15 orders of magnitude faster – essentially, in seconds.
“What was so astonishing was that no special treatment was needed: we just melted the alloy, poured it into a mold, and we had tetrataenite,” said Greer. “The previous view in the field was that you couldn’t get tetrataenite unless you did something extreme, because otherwise, you’d have to wait millions of years for it to form. This result represents a total change in how we think about this material.”
The team says this discovery could lead to a viable alternative to rare-earth magnets – although more work will be required to check whether tetrataenite created this way will work in these magnets. The research was published in the journal Advanced Science..
A new approach to producing metamaterials draws on kirigami techniques to make three-dimensional, reconfigurable building blocks that can be used to create complex, dynamic structures. Because the design approach is modular, these structures are easy to both assemble and disassemble. Kirigami, the ancient paper art of cutting, has recently emerged as a new approach to construct metamaterials with novel properties imparted by cuts. However, most studies are limited to thin sheets-based 2D kirigami metamaterials with specific forms and limited reconfigurability due to planar connection constraints of cut units. Now, 3D modular kirigami is introduced by cutting bulk materials into spatially closed-loop connected cut cubes to construct a new class of 3D kirigami metamaterials. The module is transformable with multiple degrees of freedom that can transform into versatile distinct daughter building blocks. Their conformable assembly creates a wealth of reconfigurable and disassemblable metamaterials with diverse structures and unique properties, including reconfigurable 1D column-like materials, 2D lattice-like metamaterials with phase transition of chirality, as well as 3D frustration-free multilayered metamaterials with 3D auxetic behaviors and programmable deformation modes. This study largely expands the design space of kirigami metamaterials from 2D to 3D.
Chemists have produced the first full quantum mechanical model of water — one of the key ingredients of life. The Journal of Physical Chemistry Letters published the breakthrough, which used machine learning to develop a model that gives a detailed, accurate description for how large groups of water molecules interact with one another. “We believe we have found the missing piece to a complete, microscopic understanding of water,” says Joel Bowman, professor of theoretical chemistry at Emory University and senior author of the study. “It appears that we now have all that we need to know to describe water molecules under any conditions, including ice, liquid or vapor over a range of temperature and pressure.” The researchers developed free, open-source software for the model, which they dubbed “q-AQUA.” The q-AQUA software provides a universal tool for studying water. “We anticipate researchers using it for everything from predicting whether an exoplanet may have water to deepening our understanding of the role of water in cellular function,” Bowman says. Bowman is one of the founders of the specialty of theoretical reaction dynamics and a leader in exploring mysteries underlying questions such as why we need water to live. First author of the study is Qi Yu, a former Emory PhD candidate in the Bowman Lab who has since graduated and is now a postdoctoral fellow at Yale. Co-authors include Emory graduate student Apurba Nandi, a PhD candidate in the Bowman Lab; Riccardo Cone, a former Emory postdoctoral fellow in the Bowman Lab, who is now at the University of Milan; and Paul Houston, former dean of science at Georgia Institute of Technology and now an emeritus professor at Cornell University. The discovery made the cover of the Journal of Physical Chemistry Letters. Water covers most of the Earth’s surface and is vital to all living organisms. It consists of simple molecules, each made up of two hydrogen atoms and one oxygen atom, bound by hydrogen. Despite water’s simplicity and ubiquity, describing the interactions of clusters of H2O molecules under any conditions presents major challenges. Newton’s law governs the behavior of heavy objects in the so-called classical world, including the motion of planets. Extremely light objects, however, at the level of atoms and electrons, are part of the quantum world which is governed by the Schrodinger equation of quantum-mechanical systems. Each water molecule consists of a single oxygen atom and two hydrogen atoms. “We’re about 70% water by weight,” Bowman says, “and yet, from a chemical standpoint, we don’t really understand how water molecules interact with biological systems." Although large, complex problems in the classical world can be divided into pieces to be solved, objects in the quantum world are too “fuzzy” to be broken down into discrete pieces. Researchers have tried to produce a quantum model of water by breaking it into the interactions of clusters of water molecules. Bowman compares it to people at a party clustered into conversational groups of two, three or four people. “Imagine you’re trying to come up with a model to describe the conversations in each of these clusters of people that can be extended to the entire party,” he says. “First you gather the data for two people talking and determine what they are saying, who is saying what and what the conversation means. It gets harder when you try to model the conversations among three people. And when you get up to four people, it gets nearly impossible because so much data is coming at you.”
Billions of years ago, some unknown location on the sterile, primordial Earth became a cauldron of complex organic molecules from which the first cells emerged. Origin-of-life researchers have proposed countless imaginative ideas about how that occurred and where the necessary raw ingredients came from. Some of the most difficult to account for are proteins, the critical backbones of cellular chemistry, because in nature today they are made exclusively by living cells. How did the first protein form without any life to make it? Scientists have mostly looked for clues on Earth. Yet a new discovery suggests that the answer could be found beyond the sky, inside dark interstellar clouds. A group of astrobiologists showed recently (published in Nature Astronomy) that peptides, the molecular subunits of proteins, can spontaneously form on the solid, frozen particles of cosmic dust drifting aimlessly through the universe. Those peptides could in theory have traveled inside comets and meteorites to the young Earth — and to many other worlds — to become some of the starting materials for life. The simplicity and favorable thermodynamics of this new space-based mechanism for forming peptides make it a more promising alternative to the known purely chemical processes that could have occurred on a lifeless Earth, according to Serge Krasnokutski, the lead author on the new paper and a researcher at the Max Planck Institute for Astronomy and the Friedrich Schiller University in Germany. And that simplicity “suggests that proteins were among the first molecules involved in the evolutionary process leading to life,” he said. Researchers say they’ve found a shortcut to proteins — a simpler chemical pathway that reenergizes the theory that proteins were present very early in the genesis of life. Whether those peptides could have survived their arduous trek from space and contributed meaningfully to the origin of life is very much an open question. Paul Falkowski, a professor at the School of Environmental and Biological Sciences at Rutgers University, said that the chemistry demonstrated in the new paper is “very cool” but “doesn’t yet bridge the phenomenal gap between proto-prebiotic chemistry and the first evidence of life.” He added, “There’s a spark that’s still missing.” Organic molecules are widely present in the dense interstellar medium, and many have been synthesized in the laboratory on Earth under the conditions typical for an interstellar environment. Until now, however, only relatively small molecules of biological interest have been demonstrated to form experimentally under typical space conditions. The recent paper in Nature Astronomy proves experimentally that the condensation of carbon atoms on the surface of cold solid particles (cosmic dust) leads to the formation of isomeric polyglycine monomers (aminoketene molecules). Following encounters between aminoketene molecules, they polymerize to produce peptides of different lengths. The chemistry involves three of the most abundant species (CO, C and NH3) present in star-forming molecular clouds, and proceeds via a novel pathway that skips the stage of amino acid formation in protein synthesis. The process is efficient, even at low temperatures, without irradiation or the presence of water. The delivery of biopolymers formed by this chemistry to rocky planets in the habitable zone might be an important element in the origins of life. Using common interstellar chemical species (CO, C and NH3), the authors show that peptides can be experimentally synthesized on a solid surface under interstellar conditions. The formation route circumvents the creation of amino acids in the pathway towards proteins.
A team of researchers at Columbia University have developed a new algorithm that could help quantum computers calculate molecular energy and lead to the design of new materials. The algorithm uses the most quantum bits to date to calculate ground state energy, which is the lowest-energy state in a quantum mechanical system.
The new study was published in Nature.
Via Tony Shan
Researchers have developed a rechargeable lithium-ion battery in the form of an ultra-long fiber that could be woven into fabrics. The battery could enable a wide variety of wearable electronic devices, and might even be used to make 3D-printed batteries in virtually any shape. The researchers envision new possibilities for self-powered communications, sensing, and computational devices that could be worn like ordinary clothing, as well as devices whose batteries could also double as structural parts. In a proof of concept, the team behind the new battery technology has produced the world's longest flexible fiber battery, 140 meters long, to demonstrate that the material can be manufactured to arbitrarily long lengths. The work is described today in the journal Materials Today. MIT postdoc Tural Khudiyev (now an assistant professor at National University of Singapore), former MIT postdoc Jung Tae Lee (now a professor at Kyung Hee University), and Benjamin Grena SM '13, Ph.D. '17 (currently at Apple) are the lead authors on the paper. Other co-authors are MIT professors Yoel Fink, Ju Li, and John Joannopoulos, and seven others at MIT and elsewhere. Researchers, including members of this team, have previously demonstrated fibers that contain a wide variety of electronic components, including light emitting diodes (LEDs), photosensors, communications, and digital systems. Many of these are weavable and washable, making them practical for use in wearable products, but all have so far relied on an external power source. Now, this fiber battery, which is also weavable and washable, could enable such devices to be completely self-contained. The new fiber battery is manufactured using novel battery gels and a standard fiber-drawing system that starts with a larger cylinder containing all the components and then heats it to just below its melting point. The material is drawn through a narrow opening to compress all the parts to a fraction of their original diameter, while maintaining all the original arrangement of parts.
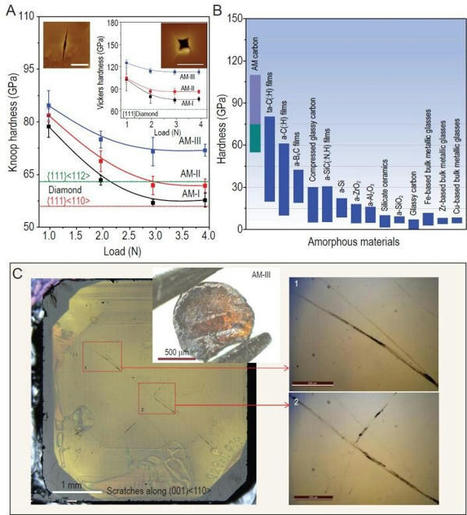
A team of researchers affiliated with a host of institutions across the globe has synthesized an AM-III carbon that is the hardest and strongest amorphous material created to date. In their paper published in the journal National Science Review, the group describes the process they used to create their new material and suggest possible uses for it. In this new effort, the researchers set out to create a new kind of glass that would be exceptionally strong. To that end, they subjected fullerenes to very high temperatures and enormous pressures and, in so doing, produced what they have called AM-III—a type of glass with crystals in it that measures higher on the Vickers hardness test than many diamonds. When looking at a diamond under a microscope, the carbon atoms and molecules that make up its crystalline structure are lined up very neatly—glass on the other hand has very little order. This difference explains why diamonds are so hard and why glass is so easily shattered. Prior research has shown that diamonds can be made by exposing graphite to high temperatures and pressure—similar to the way they are created by nature. In this new work, the researchers instead used fullerenes—structures made of carbon in the form of hollow cages. They also slowed down the process, heating and squeezing their material for approximately 12 hours, a move to prevent the material from forming into diamond. The resulting material, AM-III carbon, is yellowish, with no defined structure, and is very strong—it scored 113 giga-pascals on the Vickers hardness test, higher than some diamonds, which average just 100 giga-pascals. The researchers note that AM-III is approximately ten times as hard as steel and should be quite a bit better at stopping bullets than most vest technology. To prove its toughness, they used one sample to cut a deep scratch into a diamond. The researchers note that the toughness comes about from the material's makeup—it has micro-structures that are orderly like crystals, along with unordered glass, which makes it part glass and part crystal. It also makes the material a semiconductor with a bandgap range similar to silicon. Because of that, the researchers suggest their new material could prove useful in solar panel products.
|



 Your new post is loading...
Your new post is loading...





















Oxycodone without a prescription
Phentermine 37.5 mg for sale
Phentremin weight loss
purchase Adderall online
Where Fentanyl Patches online
Where to buy Acxion Fentermina